Abstract
Objective:Advances in 99mTc-based radiopharmaceuticals and multiple-detector gantries have the potential to increase the significance of patient motion on the diagnostic integrity of myocardial perfusion SPECT acquisitions.
Methods:An experimental study was used to evaluate the effect of various patient motions on the diagnostic integrity of myocardial perfusion SPECT data using 522 motion simulations generated from a technically and diagnostically normal dataset.
Results:Of studies with induced motion, 21.7% of simulated motion demonstrated motion-induced artifacts. Abrupt motion resulted in artifacts for 52.6% of studies, whereas bounce motion resulted in artifacts in 6.8% of studies. The locations where motion resulted in the most studies with artifacts were at 45° (36.1%) and 75° (32.4%). No statistical difference was demonstrated between single, dual-, and triple-head configurations.
Conclusion:Combining these results with those of the clinical evaluation of incidence indicates that patient motion during 99mTc-based myocardial perfusion SPECT studies is a potential source of false-positive findings for coronary artery disease. There is a 7.1% probability that myocardial perfusion SPECT studies performed at the 3 sites investigated will contain a motion-induced artifact. Fully realized, this potential results in decreased test specificity and unfavorable cost and consequence outcomes.
In recent years there have been numerous advances in the technology, science, and methodology used in performing myocardial perfusion studies in nuclear medicine. Advances in radiopharmaceuticals for the evaluation of myocardial perfusion from 201Tl-thallous chloride to 99mTc-based radiopharmaceuticals have provided advantages associated with the superior physical characteristics. Advances in technology have been responsible for the transition from planar imaging to SPECT and, more recently, to gated SPECT imaging with the emergence of multiple-detector gantries. Regardless of these advances in nuclear medicine science, patient comfort is still a concern. Consequently, patient motion is problematic with the introduction of patient motion artifacts to an image dataset presenting an interpretation dilemma.
Patient motion is a common cause of degradation of SPECT myocardial perfusion studies because SPECT requires that the object of interest remains constant for the duration of the acquisition. Motion of the heart during SPECT myocardial perfusion studies relative to the detector can create artifacts due to image alignment inaccuracies during image reconstruction (1,2). The artifacts produced by patient motion in myocardial SPECT acquisitions commonly mimic the appearances of coronary artery disease (CAD) and may be interpreted as ischemia both qualitatively and quantitatively, leading to a false-positive finding for CAD (3). In a clinical study across 3 nuclear medicine departments, we determined that 36% of clinical studies demonstrated visually detectable motion (4).
Multidetector camera configurations offer a significant advantage over single-detector systems due to decreased image acquisition times and, therefore, a decreased potential for patient motion. However, when motion does occur the effect may be compounded, with a single motion being introduced into the dataset 2 or 3 times.
The purpose of this project was to answer the research question: Is patient motion during 99mTc-based myocardial perfusion SPECT studies a source of potential false-positive findings for CAD?
MATERIALS AND METHODS
Study Design and Subjects
This simulation study was an experimental study structured to evaluate the effect of patient motion on the introduction of artifacts into the myocardial perfusion SPECT data. Each study generated with simulated motion was matched with an identically and simultaneously reconstructed control study.
Two patient studies were obtained from a nuclear medicine department patient database. Only the stress studies were used in the simulation study to capitalize on the better heart-to-background count ratio and heart-to-liver count ratio (compared with the rest studies). Similarly, both studies were chosen because the patients underwent treadmill exercise stress with high exercise tolerance rather than pharmacologic stress to minimize liver accumulation and increase heart-to-background count ratios. The 2 subjects had low pretest likelihoods of CAD and had a normal electrocardiogram response to exercise. Both patients were also lean males to reduce the possibility of physiologic artifacts. Evaluation of the patient studies indicated both to be diagnostically normal by visual and quantitative means. The absence of motion and technical artifacts was also documented visually and quantitatively.
Motion Simulation
Vertical patient motion was simulated using software to shift the selected projection or projections by the chosen number of pixels. Motions were simulated in either direction on the y-axis; however, software limitations restricted the magnitude of the motion to 1-pixel increments. In essence, vertical motions were simulated by relocating the original motion-free projection. Subject 1 (64 × 64 matrix) was used for vertical motion simulations.
Bounce motion was simulated by upward or downward vertical shifting of the raw projection data in a returning pattern, whereas abrupt motion used a nonreturning pattern—that is to say, shift for bounce simulation only required relocation of 1–3 projections, whereas abrupt motion required all subsequent projections to be relocated.
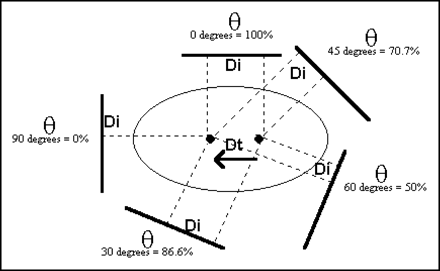
Since the direction of lateral motion is perpendicular to the axis of rotation, the apparent (imaged) motion in the projection dataset will only be a fraction of the actual motion. Figure 1 illustrates the magnitude of the actual lateral motion compared with that of the fractional apparent motion corresponding to various projections. This relationship can be described by the following equation:
 where Di is the distance of the apparent motion for projection θ and Dt is the distance of the actual motion.
where Di is the distance of the apparent motion for projection θ and Dt is the distance of the actual motion.
Schematic representation of equation Di = Dt·cos θ.
Both bounce and abrupt motions were simulated by horizontally (left and right) shifting the original motion-free projections. The magnitude of the motion was calculated individually for each projection to be translated. Increments of <1 pixel were achieved by performing the motion simulations on a study acquired in a 128 × 128 matrix (subject 2) and subsequently converting the matrix down to a 64 × 64 matrix (i.e., a 3-pixel motion in a 128 × 128 matrix provide a 1.5-pixel motion simulation in a 64 × 64 matrix).
Several variables were considered in simulating motion in the studies:
Type of motion: vertical bounce, multiple bounce, abrupt vertical and lateral or horizontal;
Gantry configuration: single, dual opposed, dual cardiac, and triple-detector simulations;
Direction of motion: vertical motions simulated in both directions on the vertical axis and horizontal motions simulated in both directions on the horizontal axis;
Number of pixels of motion (magnitude): motions for 1 pixel (small), 2 pixels (medium), and 4 pixels (large);
Number of frames to which motion was to be introduced (duration): bounce-type motions simulated with durations of 1, 2, and 3 frames before returning to their original y-ordinate and abrupt-type motions involving displacement of all remaining frames representing each detector;
Projection, or angle at which motion was to be introduced (location): starting 15° into the acquisition and at 30° intervals. Multiple bounce was represented by vertical bounce introduction at all of these locations.
A total of 522 motion simulation studies were produced as a result of combining these variables.
Reconstruction of Data
All simulation data were reconstructed simultaneously with the original motion-free study (control) using the following sequence:
Prefiltered with a Butterworth low-pass filter (order, 5.0; cutoff, 0.25);
Transverse reconstruction using a 180° filtered backprojection algorithm (right anterior oblique [RAO] to left posterior oblique [LPO]), a ramp filter, and a 15.1-cm window around the heart;
Reorientation of the transverse slices to accommodate cardiac orientation resulting in generation of short-axis, vertical long-axis, and horizontal long-axis slices of 2.4-mm thickness.
Quantitative Analysis
CEqual (Emory University, Atlanta, GA; and Cedars-Sinai Medical Center, Los Angeles, CA) quantitative analysis software was used to evaluate and compare each simulated motion dataset with the original (control) motion-free dataset for the batch. For each pair of short-axis slices, the motion simulation data were renamed as “stress” and the original motion-free data were renamed as “rest.” A motion-induced artifact should, therefore, appear as a reversible defect on the polar maps.
Statistical Analysis
The differences between independent means and proportions were calculated for a 95% confidence interval (CI). Correlation of scores was undertaken using the Cochran–Mantel–Haenszel test (5). The statistical significance was calculated using χ2 analysis for nominal data and the Student t test for continuous data. The F test analysis of variances was used to determine statistically significant differences within grouped data. P < 0.05 was considered significant. CIs without an overlap or those that did not include zero were considered to support a statistically significant difference.
RESULTS
Quantitation of Motion-Induced Artifacts
Of the studies with induced motion, 21.7% (113/522; 95% CI, 18.2%–25.2%) demonstrated motion-induced artifacts quantitatively and a total of 154 artifacts were identified. The motion-induced artifacts involved the following anatomic locations: apex, 24.0% (37/154); inferior, 20.8% (32/154); anterolateral, 9.7% (15/154); lateral, 9.7% (15/154); septum, 8.4% (13/154); apicolateral, 8.4% (13/154); and others, 18.2% (28/154). The mean extent of motion-induced artifacts was 12.5% of the myocardium. The mean severity of motion-induced artifacts was 4.3 SDs below the normal limits.
Types of Motion
Abrupt motion accounted for 77.9% (88/113; 95% CI, 70.2%–85.6%) of studies with artifacts quantitatively. Furthermore, 47.4% (37/78) of studies with vertical abrupt motion identified artifacts quantitatively, with 65.4% (51/78) for lateral abrupt studies, whereas vertical bounce, multiple bounce, and lateral bounce had 3% (7/234), 16.7% (9/54), and 11.5% (9/78) of studies identified with artifacts, respectively.
The data summarized in Table 1 demonstrate the mean percentage of the total myocardium that involves an artifact (extent) and the mean number of SDs below the normal range the artifact falls (severity). The difference between the extent and severity of artifacts between bounce motion (7.1% and 4.0, respectively) and abrupt motion (14.1% and 4.4, respectively) was 7% (95% CI, 2.7%–11.4%) and 0.4 (95% CI, −0.1 to 0.9), respectively. There was a statistically significant difference between the mean extent of artifacts between abrupt motions and bounce motion (P < 0.01); however, no statistical difference was demonstrated between the mean severity of abrupt motion compared with bounce motion (P = 0.0957).
Mean Extent and Mean Severity of Motion-Induced Artifacts for Types of Motion
Direction of Motion
No statistical difference was demonstrated in the direction of vertical bounce motion (P = 0.8272) or vertical abrupt motion (P = 0.7855). Lateral motions, however, resulted in a total of 49 artifacts for left motion and 30 for right motion, which demonstrated a statistically significant difference (P = 0.0317).
The extent of artifacts for lateral motions also demonstrated a statistically significant difference (P = 0.0206) between left motions (9.7% of myocardium) and right motions (16.4% of myocardium), with the 95% CI of the difference (6.7%) being 1.6%–11.8%. No statistically significant difference was determined (P = 0.3987) between left and right motions in relation to severity of defects.
Magnitude of Motion
Both the increase from 1- to 2-pixel magnitudes and from 2 to 4 pixels resulted in statistically significant increases in the proportion of studies with motion-induced artifacts (P < 0.01 for both). All motion-induced artifacts produced by either 1 or 2 pixel motions were identified as abrupt motions, with no artifacts being identified for bounce motions with only 1 or 2 pixel motions.
Duration of Motion
Of studies that demonstrated motion-induced artifacts, 77.9% were abrupt-type motion, whereas no artifacts were introduced in any study in which the duration was only 1 frame (Table 2). Abrupt motions resulted in the greatest extent and severity of defects.
Duration of Induced Motion vs. Number of Studies with Motion-Induced Artifacts
Gantry Configuration
Only 18.4% (43/234) of single (or dual opposed) gantry configuration motion-induced studies demonstrated artifacts compared with 25.4% (32/126) for a dual cardiac gantry and 23.5% (38/162) for a triple gantry. No statistically significant difference, however, was detected between single and dual cardiac (P = 0.059), single and triple (P = 0.107), or dual cardiac and triple (P = 0.355).
Location of Motion
Both the number of studies with motion-induced artifacts in total and with respect to the gantry configuration showed a maximum number of artifacts at 45° with the next most frequent being 75° (Table 3). No statistically significant difference was demonstrated between the proportion of studies with motion-induced artifacts for 45° and 75° (P = 0.283). The mean extent of motion-induced artifacts was greatest for motions introduced at 45° and 75°; however, no statistically significant difference was identified between the 2 angles (P = 0.6153). The mean severity (SDs below normal limits) of motion-induced artifacts was greatest for motions introduced at 45°.
Motion-Induced Artifacts with Respect to Location of Motion and Gantry Configuration
DISCUSSION
This simulation study determined that 21.7% (113/522) of the studies with induced motion were subject to motion-induced artifacts. Several investigators have reported a relationship between the presence of artifacts due to patient motion and the type, magnitude, duration, location, and direction of the motion (1,6–8). More recently, motion-induced artifacts have been reported as a function of the number of detectors in the gantry (6,9).
Abrupt motion resulted in both a greater incidence and the extent of artifacts. This study demonstrated that 77.8% (120/154) of motion-induced artifacts were for abrupt-type motions. The mean extent of artifacts for abrupt motion (14.1%) was also greater than that for bounce motion (7.1%). The nonreturning nature of the abrupt-type motion results in greater misalignment of the reconstructed data compared with that of bounce motion.
Though Cooper et al. (2) and Hendel et al. (10) reported that vertical motion has a greater impact than lateral motion on the integrity of the dataset, this study yielded discordant results. Lateral abrupt motion resulted in a greater proportion of motion-induced artifacts (65.4%; 51/78) than that of vertical abrupt motion (47.4%; 37/78) because the motion occurs in the same axis as the gantry rotation.
Although there was no difference in either the proportion of studies with motion-induced artifacts or the extent and severity of artifacts between the directions (up and down) for vertical motions, differences were demonstrated for lateral directions (left and right). Interestingly, motions in the left direction resulted in more artifacts than motions to the right (62% vs. 38%), whereas the mean extent of artifacts was greater for motions to the right (16.4% vs. 9.7%). The influence of motion laterally is complex, however; using an 180° acquisition from RAO to LPO, motion in the right direction essentially moves the heart closer to the axis of rotation, increasing resolution and maintaining a single artifact with a greater extent. Motion in the left direction, on the other hand, moves the heart further from the axis of rotation, resulting in multiple artifacts of a lesser extent.
Prigent et al. (8) reported an association between the direction of motion and the location of artifacts, with upward motion resulting in anterolateral artifacts, downward giving anteroseptal artifacts, and inferior artifacts reported for both up and down motions. This study demonstrated concordance with the findings of Prigent et al. in terms of both upward and downward motions resulting in inferior wall artifacts. Concordant results reporting upward motions producing anterolateral artifacts was described by Prigent et al.; however, this study demonstrated discordance with their observation that downward motion resulted in anteroseptal artifacts.
It was apparent in this study that both the number of motion-induced artifacts and the extent of the artifact increased with increasing magnitude (pixels) of motion (P < 0.01). It is important to note, however, that no motion-induced artifacts were identified for 1- or 2-pixel motions in bounce-type motions. Eisner et al. (3) and Cooper et al. (2) reported that, though 1-pixel motion is detectable visually, artifacts do not result until motion exceeds 2 pixels. We determined that bounce-type motion did not cause artifacts until motion was 4 pixels. Although abrupt motion was subject to introduction of artifacts from just 1-pixel motions, 1- and 2-pixel motions had a greater impact on lateral abrupt motion than on vertical abrupt motion. Once again, these results are discordant with those reported by Cooper et al. and Hendel et al. (10), that vertical motion has a greater impact than that of lateral motion.
This study demonstrated that 65.5% (74/113) of motion-induced artifacts resulted from motions occurring at 45° or 75° (these angles corresponded to anterior and LAO 45°, respectively). This observation is consistent across all gantry configurations investigated because motion occurring in projections with the greatest count density will have a greater impact on the integrity of the dataset. The anterior-to-LAO 45° projections will contain the highest count densities in myocardial perfusion SPECT since these projections have the least distance and attenuation between the heart and the detector. As expected, the mean extent of artifacts was also higher for motions occurring at anterior and LAO 45° projections.
Several investigators have reported that motion is less likely to affect the study if it occurs at the beginning or the end of the acquisition compared with motion occurring in the middle of the acquisition (2,10). This observation certainly holds true for single and dual opposed gantry configurations provided that an 180° acquisition from RAO to LPO is used (since anterior to LAO 45° projections fall in the middle of the acquisition). Multiple-detector gantry configurations and their acquisition protocols, however, complicate this observation. The dual cardiac gantry, for example, begins the acquisition at RAO 45° for detector 1 and LAO 45° for detector 2. This study draws a conclusion similar to those of Matsumoto et al. (6) and Germano (9) that motion in projections with greater myocardial count densities results in more numerous and larger artifacts.
Although there was no difference in the mean extent and mean severity of artifacts between gantry configurations, there was a difference in the proportion of studies resulting in artifacts. Only 18.4% of single (or dual opposed) detector gantry configuration motion simulations resulted in artifacts compared with 25.4% for dual cardiac and 23.5% for triple gantries. This difference was expected since a single patient motion event during acquisition will manifest as 2 events in a dual cardiac gantry dataset and as 3 events in a triple-gantry dataset while still only appearing as a single event on a single-detector gantry. Matsumoto et al. (6) and Germano (9) reported that dual gantries are more vulnerable to motion than single-detector gantries because twice the number of projections are involved. They suggested that this effect may be neutralized to some degree by the increased time required for single-detector acquisitions.
Limitations
The most significant limitation of the simulation study was the categoric evaluation of a continuous event. Although the 522 motion simulations provide a thorough cross-correlation of the parameters of interest, patient motion in the clinical setting has an infinite combination of parameters. The validity of the study is not threatened by this limitation because the purpose of the study was to identify generally applicable relationships between variables.
The simulation study identifies motion-induced artifacts using polar map quantitation. Although polar map quantitation has been validated against physician interpretation of studies, individual physicians will have differing levels of expertise in interpreting myocardial perfusion SPECT data and, thus, a motion-induced artifact detected quantitatively does not necessarily translate to a false-positive finding for CAD. They are, however, potential sources of false-positive findings for CAD.
The Research Question
The simulation study design aimed to provide a close representation of the distribution of patient motion variables determined in the clinical study (4). This facilitated the amalgamation of the outcomes of the clinical study with the simulation study providing an opportunity for decision tree analysis. Since reverse redistribution is only a problem associated with 201Tl studies, resting patient motion artifacts alone will not mimic CAD and may be readily identified as artifacts. A decision tree analysis, therefore, has been constructed for stress studies only to address the research question more precisely (Fig. 2).
Decision tree analysis of visually detectable motion and motion-induced artifacts for stress studies only. Summation of the final probabilities for A gives 7.1% probability that a stress study will have a motion-induced artifact. Summation of B gives 33.3% probability that a stress study will contain motion with no induced artifact, and C gives 59.5% probability that a stress study will have no motion.
It is apparent that there is a 7.1% probability that myocardial perfusion SPECT studies performed at 3 nuclear medicine departments will contain a motion-induced artifact that may be interpreted as CAD. Though this might seem high, Gerson (11) indicated a 10%–15% false-positive rate for CAD as a result of motion-induced artifacts. This relatively low proportion can be attributed to the fact that motion types with high incidence were associated with a low incidence of artifacts, whereas motion types with a high incidence of artifacts were associated with a low incidence of occurrence clinically.
CONCLUSION
This investigation demonstrated that patient motion during 99mTc-based myocardial perfusion SPECT studies is a potential source of false-positive findings for CAD. This potential, fully realized, not only contributes to a decrease in specificity of myocardial perfusion SPECT studies but also has unfavorable cost and consequence outcomes after coronary angiography evaluation in false-positive studies.
Acknowledgments
Part of this research was presented orally under the same title and was awarded the Mallinckrodt Award for best scientific paper by a technologist at the annual scientific meeting of the Australia and New Zealand Society of Nuclear Medicine in Sydney in May 2003. Parts of this research contributed to the Masters thesis of Janelle M. Wheat at the University of Newcastle and the authors thank Shane Dempsey and David Lyall for their editorial contribution to the same. The authors also thank the partners and staff at Central Coast Radiology for their time, expertise, and use of resources.
Footnotes
For correspondence or reprints contact: Janelle M. Wheat, BAppSc, MMedRadSc, School of Clinical Sciences, Locked Bag 588, Charles Sturt University, Wagga Wagga 2678, New South Wales, Australia.
E-mail: jwheat{at}csu.edu.au