Abstract
Objective:Two sequential 99mTc methylene diphosphonate (MDP) scans were performed on a 42-y-old woman with insulin-dependent diabetes mellitus, chronic right pyelonephritis and anemia. The initial scan showed reduced skeletal uptake with intense and diffuse hepatic uptake. Because these findings were similar to those seen when excessive hydrolyzed-reduced 99mTc colloids are present in the radiopharmaceutical, the scan was repeated after an adequate time delay. Increased skeletal uptake was evident in the second scan, but the hepatic uptake persisted. Although there are numerous causes of soft tissue activity on 99mTc MDP bone scans, the responsible pathologic entity is not always clear. This study reviews several possible reasons for such uptake, although the exact mechanism in this case remains conjectural.
Skeletal scintigraphy with 99mTc methylene diphosphonate (99mTc-MDP) is an important modality for the examination of pathologic conditions of bone. Although it is occasionally used in the evaluation of soft-tissue abnormalities, such as myositis ossificans, polymyositis, dermatomyositis, and electrical burns, extraosseous uptake is more often an unexpected finding.
Approximately 30 y ago, Francis and associates (1) proposed that diphosphonates were chemically adsorbed to the hydroxyapatite crystals of bone. This same reaction accounts for the skeletal uptake of the currently used radiolabeled 99mTc-stannous diphosphonates and analogs. The extraosseous uptake of these radiopharmaceuticals, however, cannot always be explained by the same mechanism; these agents have been observed in areas where neither active bone formation nor calcification is present. Some of the known mechanisms resulting in extraskeletal uptake include: normal variants; radiopharmaceutical preparation; interfering medications; extracellular fluid expansion; altered calcium metabolism; increased calcium content of tissues; hyperemia; altered vascular permeability; presence of iron deposits; ion exchange; adsorption onto immature collagen; and binding to denatured proteins or enzyme receptors (2).
Nonosseous uptake of bone-seeking radiopharmaceuticals is seen in a wide variety of pathologic processes involving almost any organ. Recognition of specific mechanisms and familiarity with the appearance of soft tissue abnormalities on skeletal scintigrams reduces the possibility of confusion and enhances the diagnostic value of the study. Unfortunately, the exact mechanism in many instances is yet to be completely understood. The following represents a case in which the mechanisms involved in both the extraosseous uptake and the lack of skeletal uptake can only be speculated.
CASE REPORT
An edematous 42-y-old Hispanic woman with insulin-dependent Type I diabetes mellitus, chronic right pyelonephritis and hypertension was admitted for left pleural effusion and a chest wall mass. She indicated she had been completely well until recently noting a lump on her upper left breast area that was associated with pain. Other past medical history was incomplete and vague, although she was known to have anemia (type unknown). Of interest is the fact that this patient was readmitted to the hospital approximately 6 mo later for secondary hyperparathyroidism.
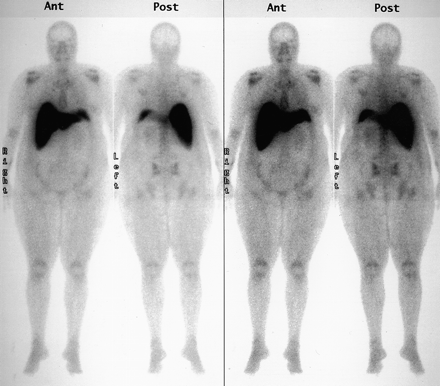
The chest wall mass was suspected to be a costochronditis seeding from the patient’s left arm subcutaneous insulin injections. Because of a family history of breast cancer, however, a 99mTc-MDP whole-body bone scan was performed 3 d after hospital admission to rule out metastatic disease. The patient was imaged after the intravenous injection of 925 MBq (25 mCi) of 99mTc-MDP and following a longer than normal delay of more than 4 h due to extensive edema and renal insufficiency. Standard anterior and posterior whole-body images were obtained on a dual-head coincidence camera (Marconi Medical Systems, Cleveland, OH) with a low-energy all-purpose (LEAP) collimator attached. Images were acquired on a 256 × 1024 matrix using a scan speed of 10.5 cm/min. This study (Fig. 1) revealed soft tissue retention, little skeletal uptake, and intense uptake in the liver and in what appeared to be a small spleen.
Initial bone scan on patient with diabetes mellitus, chronic pyelonephritis, and anemia.
A repeat bone scan was performed almost 60 h later using the same protocol (Fig. 2). Although there was considerably more skeletal uptake, hepatic radiopharmaceutical localization persisted. The activity initially seen in the splenic region was greatly diminished and was believed to correlate more to the patient’s chest wall mass. A small abnormal focus of activity was seen in the area corresponding to the approximate right anterior fourth rib that was, in retrospect, subtly noted on the first study. Additionally, symmetric increased uptake was noted around the shoulders, hips, knees and ankles.
Repeat bone scan performed approximately 60 h after the initial scan.
Pertinent laboratory tests (see Table 1 for values and normal ranges) the day of admission revealed elevated values for the following: asparatate transaminase (AST); alkaline phosphastase (ALP); blood urea nitrogen (BUN); creatinine; and glucose. Bilirubin and alanine aminotransferase (AST) were normal and the albumin was low. On the days of both scans, the patient’s serum calcium was low, phosphate was high and the calcium-phosphate products were both elevated (73.8 and 43.5, respectively, for the initial and repeat bone scans). Magnesium was measured only on the day of the first scan and was elevated.
Laboratory Results
The scintigraphic finding of abnormal activity in the hepatic parenchyma was suggestive of metastatic disease. A subsequent abdominal computerized tomogram (CT) with intravenous and oral contrast, however, revealed no hepatic abnormalities. Although not the typical location for a single metastatic lesion, the focus of activity visualized in the rib was also suggestive of metastatic disease. Subsequent radiographs of the right ribs showed no bony abnormalities. A CT-guided biopsy of the chest wall mass revealed skeletal muscle with acute and chronic inflammation and abscess formation with no malignant areas. Two foci associated with contamination from the radiopharmaceutical injection site are identified in the anterolateral abdomen on the repeat scan.
RESULTS AND DISCUSSION
The kidneys and bladder are generally the only soft tissue structures visualized on bone scans. As 99mTc diphosphonates are efficiently excreted primarily via the urinary tract, soft tissue background is usually slight, especially in younger patients with good renal function. As the patient population ages, the soft tissue background often increases, presumably due to age-related decreases in bone metabolism, osseous blood flow, and/or renal function. The extensive soft tissue background activity noted on this patient’s initial bone scan (Fig. 1) is largely due to the failure of her kidneys to excrete the radiopharmaceutical, as evidenced by the limited renal and absent bladder activity. Any disease process causing extracellular fluid expansion will also result in increased radiopharmaceutical uptake. Because she was massively edematous, some of the soft tissue background was reflective of an alteration in the extracellular fluid and subsequent tracer handling dynamics. Improper hydration during the injection-to-scan time will additionally lead to increased background activity. Although it is impossible to know for sure, this patient was supposedly compliant with the request for additional fluid intake. Inadequate hydration, however, should not be considered responsible for marked decrease in skeletal activity.
Diffuse hepatic uptake of 99mTc diphosphonates is an unusual finding in nuclear medicine practice, occurring less frequently than focal hepatic abnormalities. Table 2 lists several reported causes of diffuse hepatic uptake. Currently, the most common reason for such uptake is residual radioactivity from 99mTc sulfur colloid used the previous day for a liver scan. When that occurs, however, hepatic uptake is generally less intense than seen on these images. This patient did not have a liver scan with radiocolloid during her hospital visit.
Causes of Abnormal Diffuse Hepatic Activity with Radiolabeled Diphosphonate
Faulty radiopharmaceutical preparation was originally thought to be the cause of the abnormal distribution following the first injection of the bone agent. 99mTc colloid may be formed in the presence of excessive aluminum ion, when a pH incompatibility exists between the contents of the kit and the generator eluate, or when excessive hydrolyzed-reduced 99mTc colloid from any cause is present. This radiochemical contaminant will localize in the liver, spleen or bone marrow if it is present in sufficient quantity in the 99mTc-MDP. It is unreasonable, however, to implicate this as the sole cause since the repeat scan also revealed intense hepatic uptake. Even considering the fact that the effective half-life for colloids in the liver is relatively long (6 h), sufficient time elapsed between the 2 studies to have rendered any residual activity negligible. Radiopharmacy quality control results of the 99mTc-MDP were reviewed and found to be normal in both instances. Additionally, bone scans performed on other patients the same days did not show this abnormality.
A radiopharmaceutical error may also occur if the dose preparation-to-injection time exceeds the recommend shelf life of 6 h. In this condition, however, excessive free pertechnetate is the most likely radiochemical contaminant, not the colloid. There is little evidence of this occurrence in either image (i.e., excessive stomach, thyroid, or salivary gland uptake).
The misadministration of the wrong radiopharmaceutical [67Ga citrate or 111In labeled white blood cells (WBC)] during the first bone scan was another consideration, as a similar biodistribution is possible. This, however, was ruled out on 2 accounts. First, no patients were scheduled to receive a 67Ga citrate or a WBC study (either 111In or 99mTc labeled) that day; therefore, these radiopharmaceuticals were not on-site. Second, the whole-body bone acquisition protocol is preset to a 20% symmetric window centered around the 140 keV photopeak of 99mTc. The energies of 67Ga and 111In (93, 184, 296, 388 keV and 171, 247 keV, respectively) are such that it would be impossible to obtain an image with the sensitivity and spatial resolution of these scans.
Injection of iodinated contrast medium after administration of the bone agent was not responsible for the hepatic uptake, as no such studies occurred until after the second bone scan. As originally suggested, metastatic liver disease was virtually ruled out on the basis of a normal liver scan as demonstrated by CT. Additionally, bone agent localization is usually focal rather than diffuse and intense in hepatic metastasis.
Iron overload and various preparations for iron therapy in certain types of anemia have been associated with hepatic uptake, as well as decreased skeletal uptake in bone-agent imaging. This was first noted by Van Antwerp and associates in 1975 (3) as 99mTc phosphate activity at the site of intramuscular injections of iron-dextran. Other cases showing diffuse hepatic activity following the intravenous injection of iron colloid solutions have also been reported (4,5). It is hypothesized that a 99mTc iron-colloid complex is formed through transchelation of the MDP, yielding a compound with different organ specificity and biological fate. In this case, the new agent is taken up in the Kupffer cells of the liver. Although unsubstantiated, this patient may have been receiving iron therapy for her previously diagnosed anemia or may have had an iron-overload type of anemia—such as hemochromotosis—that is highly associated with diabetes mellitus.
Several authors (6,7,8) have documented a relationship between the degree of iron overload from iron-dextran therapy and a decrease in skeletal uptake. Additionally, Parker et al. (9) described 9 cases of iron excess due to idiopathic hemochromatosis in which the bone uptake was reduced after the administration of a bone-agent. According to Grimes and Hutt (10), the half-clearance time of iron-dextran complex, which is taken up by the reticuloendothelial system, is between 12–16 h. If this patient received iron therapy just before her hospitalization, there may have been a sufficiently high concentration of iron-dextran complex in the blood to bond with the radiopharmaceutical and cause hepatic localization and gross reduction in bone uptake. Since the patient was not receiving iron therapy during her hospitalization, the concentration would have been somewhat reduced by the time of her repeat bone scan, possibly explaining the increased skeletal uptake seen at this time.
The undetected presence of amyloidosis, another disease highly associated with renal function loss, provides a possible explanation of the abnormal distribution in these studies. Janssen et al. (11) showed that bone scintigraphy may play a very important role in the evaluation of amyloidosis when it involves the thyroid, tongue, salivary glands, nervous system, intestines, liver, spleen, or kidneys. The uptake in these soft-tissues may be so extensive that there is a reduction in skeletal activity as well. In their study, 5 of 18 patients with biopsy-proven amyloidosis had liver uptake of radiolabeled diphosphonate. It is believed that expanded interstitial volume and impaired renal function, both present in this patient, contribute to the localization of 99mTc-MDP in amyloidosis. One unique form of amyloid has been described in chronic dialysis patients (12). Due to the very limited and vague medical history, it could not be determined that this patient was on long-term dialysis, although she was dialyzed 3 d after the repeat bone scan.
Intense and diffuse liver uptake of 99mTc-MDP has also been reported in cases of severe hepatic necrosis (13). This patient’s clinical presentation and laboratory results were not consistent with severe hepatic disease.
Diffuse uptake of bone-agents in multiple organs may be the result of metastatic calcification, typically involving the joints, stomach, kidneys and lungs. It is associated with chronic renal failure and hyperparathyroidism. In hypercalcemic conditions, the solubility product for calcium and phosphate may be exceeded (Ca2+ [mg/dL] and PO43− [mg/dL] greater than 60) (14), causing a precipitation of calcium salts in the extracellular space that may be reflected by extraosseous uptake of 99mTc-MDP. This patient’s solubility product did exceed 60 during her first bone scan, but that was due to the high serum levels of phosphate, not calcium. Since metastatic calcification has consistently been associated with hypercalcemia, it is unlikely that it caused her unusual uptake.
CONCLUSION
Soft-tissue or nonosseous uptake of bone-seeking radiopharmaceuticals is not an unusual finding on skeletal scintigrams. On the one hand, there is a need for awareness of the pathophysiologic basis underlying such uptake, as it may be of critical clinical relevance in the evaluation of the patient. On the other hand, some alterations in biodistribution may be of little clinical significance, but have deleterious consequences on the quality of the bone study. Recognition of these abnormalities will reduce errors and provide important clinical information.
Acknowledgments
The author would like to sincerely thank Mr. Daniel Woods for his assistance with obtaining clinical reports on this patient.
Footnotes
For correspondence or reprints contact: Joanie MacDonald, Phone: 702-895-3136; Fax: 702-895-4819; E-mail: Macdonal{at}ccmail.nevada.edu.