Abstract
The objective was to compare estimated total blood-absorbed doses obtained by applying 4 methods to the same group of patients. In addition, these results were compared with those for the patients of other researchers, who used various other techniques over a period of more than 20 y. Methods: Twenty-seven patients (22 women and 5 men) with differentiated thyroid carcinoma were enrolled in the study. Whole-body measurements were performed as conjugate-view (anterior and posterior) counts by scintillation camera imaging. All patients received 3.7 GBq of 131I for thyroid ablation. Results: The mean total blood-absorbed doses by the first, second, third, and fourth methods in the 27 patients were estimated to be 0.46 ± 0.12, 0.45 ± 0.13, 0.46 ± 0.19, and 0.62 ± 0.23 Gy, respectively. The maximum values were 1.40, 0.81, 1.04. and 1.33 Gy, respectively. The difference between the mean values was 37.22%. In the comparison with the total blood-absorbed doses for the patients of other researchers, the difference was 50.77% (difference between the means of 0.65 and 0.32 Gy). Conclusion: None of the total absorbed doses to the blood by the 4 methods in my 27 patients was 2 Gy, the maximum permissible dose. The difference between the total absorbed doses to the blood obtained by different teams of researchers was 50.77%, whereas the difference between the values by the 4 different methods in the 27 patients was 37.22%.
The absorbed radiation dose to the blood, red bone marrow, and most organs, in the treatment of differentiated thyroid carcinoma (DTC) with radioiodine, cannot be measured directly (1). The doses to the bone marrow and blood in this procedure have been shown to be the same (2). Measurement of the absorbed dose to the blood seems to be an appropriate way to estimate the absorbed dose to the hematopoietic system and provides a better understanding of treatment quality (3,4). After introducing one of the pioneer blood dosimetry methods (5), the investigators presented a method to calculate the tolerated dose to the blood and to the target per unit of administered activity. They showed that radioiodine therapy is safe provided that the blood dose is less than 2 Gy (200 rad), the whole body retention less than 4.4 GBq (120 mCi) at 48 h, and the pulmonary uptake less than 3 GBq (80 mCi) at 24 h. In one investigation, the desirable activity ranged from 0.99 to 3.7 GBq, with acceptable results reported for ablation of tissue in DTC cases (6). Higher activity is recommended for cases of metastasis, but such doses can pose a serious risk to bone marrow and healthy tissues; therefore, activity is limited to around 7.4 GBq (4,7,8).
Past trials have associated values of 0.99–3.7 GBq with favorable outcomes in the ablation of remaining tissue in newly diagnosed cases of DTC (6). Although the optimal amount of radioiodine is debated, a study showed that the absorbed dose to the blood is a more useful predictor of ablation success in thyroid cancer patients than the amount of administered radioiodine (9).
Radiation exposure from fixed activities is very heterogeneous. Depending principally on the patient’s size and renal clearance, the calculated blood-absorbed dose per administered unit of activity (specific absorbed dose) can differ by a factor of more than 5 (10,11). A low absorbed dose to the blood might predict reduced radioiodine availability for target tissue uptake and, therefore, a low absorbed dose to the target tissue.
The aim of this study was to compare estimated total blood-absorbed doses in patients with DTC treated with radioactive iodine (who were administered 3.7 GBq for thyroid ablation) obtained by applying 4 methods on the same group of patients and by comparing these results with those for the patients of other researchers who used various other techniques. To the best of my knowledge, no studies comparing these dosimetric approaches have been published.
MATERIALS AND METHODS
Twenty-seven randomly selected patients (22 women and 5 men) with DTC were enrolled in the study. All provided informed consent to participate. Whole-body measurements were performed as conjugate-view (anterior and posterior) counts by scintillation camera imaging. All patients received 3.7 GBq for thyroid ablation. The information and data on these patients (weight, height, retention function, and residence time) were taken from Table 3 in the appendix of Hänscheid et al. (12).
To determine the specific absorbed dose, 4 methods were applied.
First Method
The first method was considered the standard one. Whole-body probe measurements and blood collections (2-mL whole-blood samples) were conducted 2, 6, 24, 48, and 72–96 h after the administration of 131I to obtain time–activity curves. The following equation was applied (13): Eq. 1where Dblood is the absorbed dose, A0 is the initial administered or received activity,
Eq. 1where Dblood is the absorbed dose, A0 is the initial administered or received activity,  is total-body residence time,
is total-body residence time,  is the residence time in a milliliter of whole blood, h is the patient’s height in centimeters, and m is the patient’s mass in kilograms.
is the residence time in a milliliter of whole blood, h is the patient’s height in centimeters, and m is the patient’s mass in kilograms.
Second Method
The second method estimates the specific absorbed dose from external whole-body counting without blood sampling. The following equation was applied (14):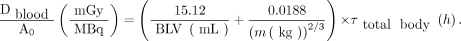 Eq. 2
Eq. 2
The individual blood volume (BLV) can be estimated from the patient’s mass and height (15), where BLV = 31.9 × h + 26.4 × m − 2,402 for men and BLV = 56.9 × h + 14.1 × m − 6,460 for women.
Third Method
The third method was applied in cases of markedly low or high 48-h whole-body uptake. The following equation used the 48-h whole-body retention measured in a diagnostic assessment to adapt the activity in the subsequent radioiodine therapy (3,16): Eq. 3
Eq. 3
Fourth Method
In the fourth method, the specific absorbed dose to the blood was calculated by applying a refined method (12,14): Eq. 4
Eq. 4
Total absorbed dose was calculated by applying the following equation: Eq. 5Also, the tolerable activity for a blood-absorbed dose of 2 Gy was calculated by the following equation (2):
Eq. 5Also, the tolerable activity for a blood-absorbed dose of 2 Gy was calculated by the following equation (2):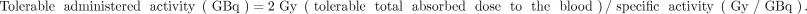 Eq. 6
Eq. 6
Other techniques and methods have been introduced and applied by different groups of researchers (4,12,17–20) to calculate the total absorbed dose. I compared my results with those obtained by the others, as shown in Table 1.
Comparison of Mean Total Absorbed Dose with 3.7 GBq of Administered Activity in Prior and Present Investigations
RESULTS
The patients’ mean height and mass (±SD) were 163.41 ± 9.92 cm and 70.33 ± 14.25 kg, respectively. On the basis of their height and weight, the patients’ individual blood volume was estimated at 5,439.16 ± 703.62 mL for men and 3,575.01 ± 440.01 mL for women.
Figures 1 and 2 demonstrate the relationship between the blood-absorbed dose by the first, second, and fourth methods. Blood-absorbed dose calculations were further analyzed according to the Bland–Altman method, which is a supplemental test to compare 2 methods when the true value is unknown (Figs. 3 and 4). Of the values in the study, 93% (25/27) for the first and second methods and 96% (26/27) for the first and fourth methods were within the limits of agreement.
Scatterplots of blood-absorbed doses by first and second methods.
Scatterplots of blood-absorbed doses by first and fourth methods.
Scatterplots of difference in blood-absorbed doses by plotting first and second methods, as y-axis, against mean absorbed doses by first and second methods, as x-axis (Bland–Altman analysis). Solid line indicates mean difference between first method and second methods, and dotted lines indicate 95% limits of agreement (mean ± 1.96 SD).
Scatterplots of difference in blood-absorbed doses by plotting first and fourth methods, as y-axis, against mean absorbed doses by first and fourth methods, as x-axis (Bland–Altman analysis). Solid line indicates mean difference between first method and fourth methods, and dotted lines indicate 95% limits of agreement (mean ± 1.96 SD).
The estimated total absorbed doses (Gy) to the blood by the 4 methods in the 27 patients are shown in Table 2. Absorbed dose was significantly higher with the fourth method than with the first one. A strong positive association (n = 27; R = 0.83) was found between the first method (standard) and the second method from one part and R = 0.99 between the first and fourth method from the other part (Figs. 1 and 2). There was no significant difference between the blood-absorbed doses by the first and second methods (P < 0.05). Significant differences were found between the blood-absorbed doses by the first and fourth methods (P > 0.05).
When the total absorbed doses by the 4 techniques were compared (Table 1), the difference between the average values ranged from 27% (comparison between the fourth and the first methods) to 1.79% (comparison between the second and the first methods). The values by the first method ranged from 0.23 to 1.04 Gy, whereas the values by the fourth method ranged from 0.33 to 1.33 Gy.
Table 1 includes the total absorbed doses obtained or calculated by the other teams of researchers.
DISCUSSION
Higher activity levels of radioiodine are more likely to ablate remnants and destroy residual micrometastases than lower levels (15). With high-dose therapy, the dose to the blood should be less than 2 Gy to reduce bone marrow toxicity. In recommended protocols for DTC therapy, radioiodine ablation has long been associated with a lower rate of recurrence and distant metastases, as well as a lower risk of cancer mortality, than undergoing only surgery (10,21–29). A study of 30-y recurrence rates in DTC patients treated with radioiodine ablation after their thyroidectomies found no significant difference between low-dose (1–1.85 GBq) and high-dose (1.89–7.4 GBq) groups (22).
One of the most obvious reasons for increasing the blood dose of one patient in comparison to other patients with the same administered activity is a high level of residence time activity to the blood and whole body that produces the area under the time–activity curve and the blood dose (4,12). Many studies have been performed to find the proper administered activity of radioiodine for the treatment of DTC (12,24). The absorbed dose to the patient’s blood did not significantly differ between a 3.7-GBq and a 4.62-GBq radioiodine treatment. However, the absorbed dose to the patient’s blood did significantly differ between a 3.7-GBq and a 5.55-GBq treatment (4).
In my study, the maximum values by the first, second, third, and fourth methods were 1.04, 0.81, 1.04 and 1.33 Gy, respectively. None of the total absorbed doses to the blood by the 4 methods for the 27 patients was 2 Gy, the maximum permissible dose. When comparing the total absorbed doses to the blood for the patients of the other researchers (Table 1), I found a difference of 50.77% (the difference between 0.65 and 0.32 Gy) (4,25). The mean total blood-absorbed doses by the 4 techniques were estimated to be 0.46 ± 0.12 Gy, 0.45 ± 0.13 Gy, 0.46 ± 0.19 Gy and 0.62 ± 0.23 Gy, respectively, with an administered activity of 3.7 GBq.
When comparing the average estimated total blood-absorbed doses by the second, third, and fourth methods with those by the first method, I found that these values were, respectively, 1.89% less than that by the first method and 0.79% and 37.22% more than those by the first one. I prefer applying the second method (14) because it can estimate the blood dose from external whole-body counting without blood sampling and with high accuracy compared with the first method (the difference between the average values by the first and second methods is <2%). In addition, the residence time in this method is deduced from retention of all points that represent the time–activity curve from 2 to 96 h and not from retention of a single point (48 h), as used by the third method.
The blood-absorbed doses by the fourth method are highly overestimated compared with those by the first one. Overestimated absorbed radiation doses in any medical application are undesirable, as they tend to lead to exaggerated radiation protection procedures that may be far from necessary or justified. Errors or uncertainties in measurements can be reduced by taking greater care with them, repeating them, using reliable instruments, and properly calibrating the instruments.
CONCLUSION
None of the total absorbed doses to the blood by the 4 methods for the group of 27 patients was 2 Gy, the maximum permissible dose. The difference between the total absorbed doses to the blood obtained by different teams of researchers was 50.77%, whereas the difference between the values by the 4 methods in the 27 patients was 37.22%. I prefer applying the second method (14) because it can estimate the blood dose from external whole-body counting without blood sampling and with high accuracy compared with the first method.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is the method by which total blood-absorbed dose is calculated important in ablation treatment of patients with DTC?
PERTINENT FINDINGS: In the other studies, the difference between total absorbed doses to the blood was 50.77%, whereas in the current study, the difference using the 4 methods was 37.22%. Blood-absorbed doses did not significantly differ between the first and second methods (P < 0.05) but did significantly differ between the first and fourth methods (P > 0.05).
IMPLICATIONS FOR PATIENT CARE: The significant differences in absorbed doses found by different researchers indicates that the choice of method to determine the absorbed dose is important. The second method saves time, is easier for staff and patients, and is nearly as accurate as the first method.
Footnotes
Published online Jul. 11, 2023.
REFERENCES
- Received for publication November 20, 2022.
- Revision received May 1, 2023.