Abstract
The objective of this study was to develop instant thin-layer chromatography (ITLC) conditions for the determination of radiochemical purity of 68Ga-DOTATATE in a shorter time period than those stated in the NETSPOT (Advanced Accelerator Applications, Saint-Genis-Pouilly, France; AAA) kit package insert (PI). A faster ITLC system is needed to reduce the 48- to 50-min development time so that more radioactivity is available for single patient use and wait times are shorter in the event of kit failure. Methods: Variations of the PI mobile system were evaluated with microfiber chromatography paper impregnated with silica gel (ITLC-SG). After a more suitable mobile system was identified, evaluation began by attempting to shorten the 10-cm development distance to 7, 8, and 9 cm. Results: Experiments using variations of PI mobile phase showed that increasing the proportion of methanol in the mobile phase decreased development time. Additionally, if the ratio of 1 M ammonium acetate was reduced to 10% or less, retention factor values fall outside specification. Reducing the development distance shortened development time as expected; however, it also affected the resolution aspect of the radiochromatogram. Conclusion: The fastest developing ITLC system, which maintained resolution and peak shape, was methanol:1 M ammonium acetate (80:20 V/V) with ITLC-SG using a development distance of 8 cm.
- NETSPOT
- 68Ga-DOTATATE
- instant thin layer chromatography (ITLC)
- radiochemical purity (RCP)
- positron emission tomography (PET) drug
On June 1, 2016, the Food and Drug Administration approved NETSPOT (Advanced Accelerator Applications [AAA]), a kit for the preparation of 68Ga-DOTATATE injection. This radioactive drug is indicated for the localization of somatostatin receptor–positive neuroendocrine tumors in adult and pediatric patients (1). The package insert (PI) for NETSPOT calls for radiochemical purity (RCP) determination using glass microfiber chromatography paper impregnated with salicylic acid (ITLC-SA) (1). However, this ITLC-SA method takes approximately 50 min to complete. The long development time lengthens the overall drug preparation time and therefore PET imaging operations.
A PET imaging area is a fast-paced working environment that often has a regimented schedule. A disrupted schedule leads to longer waiting times for patients, who may themselves have tight clinic schedules. A significant amount of radioactivity is also lost via radioactive decay using the ITLC method in the PI. When a 68Ga generator is getting weaker, this loss of radioactivity becomes an increasingly visible issue.
A more rapid ITLC method is needed to determine the RCP of 68Ga-DOTATATE. A shorter RCP time would maximize the amount of 68Ga-DOTATATE available for patient use and shorten waiting times in the event of, for example, a NETSPOT kit failure. Therefore, the objective of this study was to develop ITLC conditions for determination of the RCP of 68Ga-DOTATATE in a shorter time period than that stated in the PI (1).
MATERIALS AND METHODS
Radiolabeling of 68Ga-DOTATATE
NETSPOT comes with a reaction vial containing lyophilized DOTATATE (40 μg); 5 μg of 1,10 phenanthroline; 6 μg of gentisic acid; 20 mg mannitol; and a buffer vial of reaction buffer solution (∼1 mL) containing 60 mg of formic acid, 56.5 mg of sodium hydroxide, and water for injection (1,2). A 0.2-μm vent filter assembly and a needle, attached to a 0.22-μm vented filter and 68Ge trapping cartridge (660 mg of porous silica), connected to a 68Ge/68Ga generator (Galliapharm; Eckert & Ziegler Radiopharma GmbH) are inserted into the reaction vial septum within a lead pig. DOTATATE is radiolabeled by eluting the 68Ge/68Ga generator with 5 mL of 0.1 M sterile ultra-pure HCl eluant (Eckert & Ziegler Radiopharma GmbH) (1,2).
The PI dictates that processing take place in an aseptic environment; therefore, all production hardware and all processing take place within an ISO 5 A2 PET Dispensing Isolator (AMERCARE LTD; Thame) (1,2). Immediately after elution, 0.5 mL of the NESPOT kit buffer is added to the reaction vial, followed by heating (1,2). The heating portion of the drug labeling is completed using heater block for 8 min at 98°C, the temperature set point (1,2). The heater wells are protected from the flows inside the hood using an in-house fabricated Plexiglas cover. After heating, the reaction vial is cooled for 10 min per the PI (1,2). After cooling, a small sample is withdrawn from the reaction vial for quality control (QC), which includes pH testing, visual inspection, radionuclidic identity, membrane filter integrity, bacterial endotoxins test, retrospective sterility testing, and RCP testing (1,2).
RCP Testing Methods
The NETSPOT PI calls for methanol:1 M ammonium acetate (50:50 V/V) as the mobile phase using ITLC-SA chromatography paper (1). In January 2017, AAA amended the PI to include glass microfiber chromatography paper impregnated with silica gel (ITLC-SG) using the same mobile phase conditions (2). ITLC-SG offers a faster development time (3). A sample was spotted 1 cm from the bottom of the strip using a capillary syringe and developed a distance of 10 cm from the point of application, within glass development tanks and 3–4 mm of mobile phase.
After ITLC development, RCP and retention factor (Rf) values were determined by scanning strips with a radiometric ITLC scanner (AR2000; Eckert & Ziegler Radiopharma GmbH). Rf is defined as the ratio of the distance traveled (determined by measurement of a radio-peak centroid) to the distance traveled by the solvent (solvent front). Rf values and RCPs were calculated by identification and integration of chromatogram peaks. RCP acceptance criteria in the PI are 68Ga-DOTATATE ≥ 92% and other Ga species ≤ 5% (1,2). Chromatograms were generated for each ITLC system tested. Two chromatogram examples of runs using methods in the updated PI (2) and the final optimal conditions are illustrated in Figure 1.
Radiochromatograms of methanol:1 M ammonium acetate (50:50 v/v)/ITLC-SG system with a development distance of 70 mm (top) and methanol:1 M ammonium acetate (80:20 v/v)/ITLC-SG system with a development distance of 80 mm (bottom). Dotted lines represent origin and solvent front, respectively.
Preparations of Tested Samples
Samples used to spot strips are categorized into 2 groups: 68GaCl3 (generator eluate; free 68Ga) and ≥95% RCP 68Ga-DOTATATE.
Acceptable RCP Value
An RCP of 95% was selected as the minimum acceptance limit because to pass the NETSPOT RCP specification, the total 68Ga species (other than the intended labeled product), must be in a total concentrations of ≤5%. In actuality, the NETSPOT PI states that ≥92% 68Ga-DOTATATE is needed to pass the RCP specification (1,2). In the case of a sample with an RCP of 92%, a single radiochemical impurity making up 8% of the sample would violate the acceptance criteria that a single 68Ga species must be ≤5% (1,2).
Tested Stationary Phase
ITLC-SG was used in our study due to its faster development time (3).
Tested Mobile Phases
Various mobile phases were tested, which are described in Table 1.
RCP Evaluation Outcomes with Various Mobile Phases
Tested Conditions for ITLC Methods
Two criteria need to be met by an ITLC system to fulfill the objective of this study. The system needs to develop more rapidly relative to the conditions used for the PI and revised PI. Also ITLC system radiochromatography must have sufficient resolution (Rs) between radioactive species.
All tested conditions are depicted in Tables 1 and 2. ITLC-SG was investigated in combination with a wider selection of mobile phases once it was determined that ITLC-SG development time was generally faster.
Rs is included in Table 1 as a metric for ITLC system performance. Rs is calculated using the following equation (4):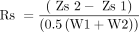 Zs2 and Zs1 are the migration distances of the 2 components, and W1 and W2 are the corresponding widths at the bases of the peaks. Rs = 1.25 is sufficient for quantitative measurements using radio–thin-layer chromatography. In the case of fronting or tailing peaks, Rs ≥ 2.5 is required for reliable quantification.
Zs2 and Zs1 are the migration distances of the 2 components, and W1 and W2 are the corresponding widths at the bases of the peaks. Rs = 1.25 is sufficient for quantitative measurements using radio–thin-layer chromatography. In the case of fronting or tailing peaks, Rs ≥ 2.5 is required for reliable quantification.
Comparison of Standard and Alternative RCP Testing Methods
Alternative Development Distances
It was anticipated that the developing time would be reduced if the development distance was shortened. Various development distances were tested (Table 2). Nevertheless, we were interested in finding out whether the ITLC-SG with a shorter development distance also provided a suitable Rs on top of time reduction.
RESULTS
Mobile Phases
Results of various mobile phases are shown in Table 1. The ITLC-SG system with methanol:1 M ammonium acetate (80:20 V/V) scored a relatively high mark in each of the 4 evaluated elements (i.e., radiochemical impurities Rf, 68Ga-DOTATATE RCP, development time, and Rs). The Rf value of generator eluate (i.e., free 68Ga) was predominantly located at the baseline (i.e., Rf < 0.1).
Alternative Development Distances
As shown in Table 2, the PI RCP testing method with a 7-cm development distance had the shortest development time in the group of methanol:1 M ammonium acetate (50:50 V/V). The Rs value for this system was acceptable (i.e., 1.5) (Table 2).
Although the 7-cm RCP testing system with 80:20 V/V methanol:1 M ammonium acetate was the fastest of all tested samples, its resolution power was not adequate (i.e., Rs = 0.8) (Table 2).
Judging from the development time and resolution capability, the optimized conditions of this evaluation was the RCP testing method with 8-cm development distance and mobile phase of methanol:1 M ammonium acetate (80:20 V/V) (Table 2).
DISCUSSION
Reducing quality control time by shortening the RCP test is important because as a 68Ga generator ages, less 68Ge parent is available, leading to reduced output of 68Ga. After 9 mo, the quantity of 68Ge will be reduced by about 50% (5). Additionally, a 68Ga generator requires about 7 h to achieve full yield after an elution according to the summary of product characteristics (5). In the event of a kit failure, maximizing available radioactivity after a second elution, before the generator achieving its full yield, becomes critical.
In the effort to reduce time of the RCP test, widely used stationary phases were selected, which were able to separate many classes of compounds (6). It was hypothesized that 68Ga-DOTATATE, with its amide, amine, and hydroxyl functional groups, would adsorb more tightly to the acid structure of salicylic acid than it would to the silica gel structure (6).
In their revision to the PI (2), AAA proved the new mobile phase conditions offered faster development time. Broadly, as methanol concentration increases development time decreases while sample component separation efficiency also decreases. ITLC systems without ammonium acetate or mobile phases with 1 M ammonium acetate concentrations ≤ 10% produce undesirable chromatograms. The ammonium acetate modifier is likely increasing efficiency of 68Ga-DOTATATE separation from other 68Ga species, resulting in more symmetric, well-defined peaks (7).
The results in Table 1 demonstrate that methanol:1 M ammonium acetate (80:20 V/V)/ITLC-SG was the quickest developing ITLC system with Rs sufficient to determine RCP. The results in Table 2 show that RCP test duration may be decreased by reducing ITLC development distance. Acceptable Rs can be achieved with a development distance range of 7–10 cm for methanol:1 M ammonium acetate (50:50 V/V)/ITLC-SG, and 8–10 cm for methanol:1 M ammonium acetate (80:20 V/V)/ITLC-SG (Table 2).
A development distance of 8 cm will result in the fastest time for this test under these conditions. The Rs of this system was only slightly lower (1.4 vs. 1.5) than those with the speediest PI RCP testing method with a 7-cm development distance (Table 2). When compared with the most current AAA ITLC-SG conditions (3), there is an approximate 14-min decrease in development time (Table 2).
CONCLUSION
The optimal ITLC conditions for the determination of RCP was methanol:1 M ammonium acetate (80:20 V/V)/ITLC-SG using a development distance of 8 cm. These conditions resulted in a development time of 12.7 ± 0.2 min.
DISCLOSURE
Financial support for this study was provided by the Mayo Clinic PET Radiochemistry Facility, Rochester, Minnesota. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online May 3, 2018.
- Received for publication August 14, 2017.
- Accepted for publication April 5, 2018.