Abstract
Glomerular filtration rate (GFR) is the best indicator of renal function. The gold standard for GFR measurement is inulin clearance. However, its measurement is inconvenient, time-consuming, and costly. Thus, in both scientific studies and routine clinical practice nuclear medicine methods (99mTc-diethylenetriaminepentaacetic acid [99mTc-DTPA] and 51Cr-ethylenediaminetetraacetic acid [51Cr-EDTA]) are preferred, and they correlate strongly with inulin clearance. In addition, cystatin C and β-trace protein have also recently been used for this purpose. In the literature, however, data are limited about the clinical value of cystatin C and β-trace protein in GFR measurement in chronic renal disease (CRD), and the results have been inconclusive. In this study, we aimed to determine the efficiency of cystatin C and β-trace protein in the determination of GFR in CRD patients. Methods: Eighty-four patients with CRD were included in the study (59 men and 25 women; age range, 21–88 y; mean age, 61 y). GFR was calculated using the gold-standard 99mTc-DTPA 2-sample plasma sampling method (TPSM) and 2 alternative methods: a formula using cystatin C and a formula using β-trace protein. The correlation between TPSM and the cystatin C and β-trace protein methods was assessed, and Bland–Altman analysis was used to graph scatterplots of the differences at a confidence interval of 95% (mean difference ± 1.96 SDs). Results: GFRs calculated using both alternative methods correlated strongly with those calculated using the gold standard. However, the correlation was stronger for the cystatin C method than for the β-trace protein method, and neither method produced reliably consistent GFRs. Conclusion: This study demonstrated that cystatin C and β-trace protein do not reflect GFR with sufficient accuracy.
Chronic renal disease (CRD) is a nephrologic syndrome secondary to chronic, progressive, and irreversible nephron injury due to various causes. CRD is distinguished from acute renal injury by azotemia for more than 3 mo, long-standing uremic signs and symptoms, signs and symptoms of renal osteodystrophy, anemia, hyperphosphatemia, hypocalcemia, large cylinders in urine sediment, and bilaterally small kidneys as seen on radiologic examination (1).
Evidence of renal injury may be structural or functional and can be obtained from kidney biopsy, urinalysis, blood tests, and imaging examinations. The most common and most easily detected indicator of kidney injury resulting in glomerular dysfunction is proteinuria. According to the definition of the National Kidney Foundation, a reduction in glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 is sufficient to diagnose CRD, even when there are no signs of renal injury (1,2).
GFR is the most important diagnostic tool for global assessment of renal function. With measurement of GFR, it is possible to detect renal injury and its severity and, through serial measurements, to assess the progression of renal disease. Total GFR is the sum of the filtration rates of each functioning nephron. The normal GFR depends on a person’s age, sex, and body habitus. Showing great variability even among healthy persons, GFR is approximately 130 mL/min/1.73 m2 in men and 120 mL/min/1.73 m2 in women (1).
Various methods are used to calculate and assess GFR. Inulin clearance, the accepted gold standard, is the most accurate. However, its difficult application, high cost, low availability, and long measurement time hamper its routine use in clinical practice (3). In the past, evaluation of renal function has been based substantially on serum creatinine measurement. Endogenous creatinine clearance is a widely accepted method, but it has a weak relationship to inulin clearance and, because it is secreted from tubuli, may lead to erroneous results in patients with reduced renal function.
In the measurement of GFR, blood sampling after injection of 99mTc-diethylenetriaminepentaacetic acid (99mTc-DTPA) and 51Cr-ethylenediaminetetraacetic acid (51Cr-EDTA) is widely used because of ease of application and high accuracy (4,5). Blaufox et al. (6) have demonstrated that measurement of 51Cr-EDTA clearance gives the closest results to inulin clearance. It has also been shown that 51Cr-EDTA clearance agrees with 99mTc-DTPA clearance and that 99mTc-DTPA can be substituted for 51Cr-EDTA (7). Furthermore, Rehling et al. (8) have shown that 99mTc-DTPA clearance is very close to inulin clearance, the accepted gold standard.
Cystatin C is a nonglycosylated low-molecular-weight protein found in human tissues and biologic fluids. It is carried unbound to any protein in plasma; it is reabsorbed from the proximal tubule and metabolized but not secreted; and it is unaffected by nonrenal parameters such as age, sex, diet, and muscle mass (9). Its use as an endogenous marker of GFR measurement as an alternative to creatinine has been demonstrated (10,11).
The literature indicates that cystatin C does not accurately reflect GFR in patients undergoing chemotherapy (12,13). Aydin et al. (12) and Knight et al. (13) have shown that, because of the nephrotoxic effects of chemotherapeutic drugs, cystatin C does not accurately reflect GFR in patients receiving immunosuppressive therapy. The nephrotoxic effects of chemotherapeutic drugs induce tubular cell injury, which impairs reabsorption and catabolism of cystatin C from the proximal tubules. In contrast, we previously showed that cystatin C significantly correlates with the 99mTc-DTPA 2-sample plasma sampling method (TPSM) in reflecting GFR in patients not undergoing chemotherapy (12). However, to our knowledge there are no studies in the literature specifically investigating whether cystatin C can be used as a marker of GFR in patients with renal dysfunction accompanied by varying degrees of tubular injury and not receiving any chemotherapy, steroid therapy, or other medical therapies.
One alternative method of determining GFR is the recently introduced serum β-trace protein. β-trace protein is a low-molecular-weight enzyme that belongs to the prostaglandin-D synthase group. It is found in all tissues of the human body with the exception of the ovaries. It is almost completely filtered via the kidneys. Some studies have indicated that the β-trace protein cystatin C may be used as a marker of GFR in adults (14). Unfortunately, few studies have investigated GFR measurement using β-trace protein in CRD, and their results have been controversial.
Moreover, to our knowledge there have been no studies of the ability of GFR calculated using cystatin C and β-trace protein to reflect renal function in CRD patients (14), nor are there studies of the correlation and agreement between cystatin C, β-trace protein, and TPSM in such patients. Thus, our study, which we believe to be the first in this regard, should be a major contribution to the existing literature.
N-acetyl-b-d-glucosaminidase (NAG), a highly sensitive marker of renal tubular injury, is the most studied urinary enzyme and has the widest range of uses. Although NAG is present in all segments of the nephron, it is particularly abundant in the lysosomes of the proximal renal tubular cells and is used to determine the function of that region (15,16). Its high molecular weight (130,000–140,000 Da) prevents its passage through the glomerular basal membrane; thus, its daily excretion is highly stable despite minimal diurnal fluctuations. NAG is found in trace amounts in the urine of healthy persons, and it is a sensitive test of the severity of renal injury before function becomes impaired. NAG activity correlates with disease activity. In addition to its high sensitivity, NAG is a good marker of renal injury because it shows an increase in urine earlier than other methods and its increase parallels the severity of pathologic changes. Thus, NAG has found a place for various renal conditions (17).
β2 microglobulin is a nonglycosylated peptide protein with a low molecular weight of 11,800 Da. It is found on the surface of all cell types as a continuous part of the light chain of the major histocompatibility complex of class I antigens. Its endogenous production is fairly stable, and it is filtered easily and almost solely by the glomeruli. Approximately 99% of the protein is reabsorbed from the proximal tubules via pinocytosis and metabolized. Hence, β2-microglobulin levels are constant in healthy individuals. In patients with CRD, the level of GFR reduction relates to the level of β2-microglobulin accumulation in the blood. With a reduction in GFR, β2 microglobulin starts to accumulate in the blood, and the increase in β2 microglobulin occurs earlier and is more pronounced than the increase in serum creatinine. It has been reported that β2 microglobulin is a better marker than serum creatinine for calculating GFR and showing renal dysfunction (18–21).
In the present study we aimed, first, to show the ability of cystatin C and β-trace protein to reflect renal function in CRD patients, with TPSM used as the gold standard, and second, to study the correlation between the severity of tubular function injury (as assessed by NAG and β2-microglobulin levels) and cystatin C and β-trace protein levels. The local ethics committee at Akdeniz University School of Medicine approved the study, which was also a project of the Akdeniz University Scientific Research Coordination Unit (project 2011.01.0103.012).
MATERIALS AND METHODS
Patients
This study enrolled a total of 84 patients with CRD (59 men and 25 women; mean age, 61 ± 12 y; age range, 21–88 y) who were under follow-up by the Department of Nephrology at Akdeniz University School of Medicine. Sixteen patients (9 men and 7 women) were excluded because they were taking steroid therapy or chemotherapy or had thyroid dysfunction (because cystatin C level is altered by thyroid dysfunction).
Those patients who regularly kept their follow-up appointments were informed about the study protocol, and patients who were willing to participate gave written informed consent. Each underwent spot urine and blood sampling and, on the same day, GFR measurement using TPSM. The participants were hydrated under physiologic conditions starting 30 min before and ending 4 h after injection of 99mTc-DTPA (74–111 MBq; binding efficiency ≥ 95%).
Laboratory Tests
Venous blood samples (5 mL) were drawn from the non–99mTc-DTPA-injected arm into heparinized tubes at 120 and 240 min after injection. The samples were immediately centrifuged at 2,000g (4,000 rpm) for 5 min, the plasma was separated, and the serum was stored at −80°C for 3 mo and thawed on the day of analysis.
The spot urine sample was used first for full urinalysis and then for measurement of cystatin C, NAG, β2 microglobulin, sodium, phosphate, and creatinine.
A BN II nephelometry device (Siemens Healthcare Diagnostics Ltd.) was used to analyze serum and urinary cystatin C and β2-microglobulin (with the nephelometric method). A Cobas 8,000 autoanalyzer (Roche Diagnostics) was used to analyze serum blood urea nitrogen (with the enzymatic, colorimetric method), serum creatinine (with the rate-blanked, compensated Jaffe method), serum phosphate (with the colorimetric method), serum and urinary sodium (with the indirect ion selective electrode method), and urinary protein (with the turbidimetric method).
Serum β-trace protein was analyzed using the solid-phase sandwich ELISA method (human prostaglandin D synthase [lipocalin-type] ELISA kit; BioVendor). In this method, microplates containing 96 wells precoated with the polyclonal anti–β-trace protein antibody were used. The 100-times diluted serum and β-trace protein found in the standards, which were added to the wells, was bound to these antibodies after a 1-h incubation, and the unbound parts were removed by repeated washing. Next, a conjugate was added to the medium and a further 60-min incubation was allowed. The unbound enzyme was removed from the medium by washing, and the substrate was added. The density of the color after 10 min of incubation was measured at 450 nm. The amount of β-trace protein in serum samples was calculated using the curve drawn with the help of the standards. The results are presented as ng/mL.
Urinary NAG was analyzed using the colorimetric method (Diazyme kit). The results are presented as IU/L.
Serum-free T4 and thyroid-stimulating hormone were measured using the electrochemiluminescence immunoassay method in the Cobas 8,000 autoanalyzer.
GFR Measurement Methods
GFR was measured in compliance with the guidelines of the Turkish Society of Nuclear Medicine Nephrourology Task Group (22), using 3 methods. In the first, the gold standard, TPSM was calculated using the following formula (6,23): where D is total injected dose (cpm), P1 is plasma activity at time T1 (cpm/mL), P2 is plasma activity at time T2 (cpm/mL), T1 is 120 min, and T2 is 240 min. The value obtained by this formula was corrected using the method of Bröchner-Mortensen et al. (24). All values obtained using this correction were subjected to a body-surface-area correction of 1.73 m2 to obtain the final GFR (25).
where D is total injected dose (cpm), P1 is plasma activity at time T1 (cpm/mL), P2 is plasma activity at time T2 (cpm/mL), T1 is 120 min, and T2 is 240 min. The value obtained by this formula was corrected using the method of Bröchner-Mortensen et al. (24). All values obtained using this correction were subjected to a body-surface-area correction of 1.73 m2 to obtain the final GFR (25).
In the second method, GFR (mL/min/1.73 m2) was calculated using cystatin C level (mg/L) with the formula of Hoek et al. (26):
In the third method, GFR (mL/min/1.73 m2) was calculated using serum β-trace protein level (mg/L) with the formula of Pöge et al. (27).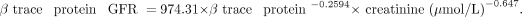
The patients were categorized into 3 of the groups proposed by the Kidney Disease Outcomes Quality Initiative (KDOQI) (9), based on GFRs calculated using TPSM: 0–15 mL/min/1.73 m2 (group 1), 15–30 mL/min/1.73 m2 (group 2), and 30–60 mL/min/1.73 m2 (group 3) (although the KDOQI criteria include 5 groups, we used only 3 groups since few subjects had a GFR of 60 mL/min/1.73 m2 or above).
Correlation and agreement within a 95% confidence interval were studied between the cystatin C and β-trace protein levels and between GFRs calculated with these parameters and those calculated with TPSM. In addition, the correlation was studied in patients with versus without renal tubular injury by categorizing patients into groups according to NAG and β2-microglobulin levels, which indicate tubular injury at an early stage: NAG ≤ 6.1 and NAG > 6.1; β2 microglobulin ≤ 0.2 mg/L and β2 microglobulin > 0.2 mg/L.
Statistical Analysis
Statistical analyses were performed with SPSS, version 18.0 (IBM), and MedCalc, version 8.2.0.1. All results are presented as mean ± SD. The correlation between GFRs calculated using TPSM and GFRs calculated using cystatin C, β-trace protein, and β2-microglobulin was analyzed using Spearman rank correlation. The correlation was also analyzed between the GFRs for groups 1–3 grouped according to the TPSM and the GFRs calculated with the other methods.
Bland–Altman analysis was used to determine the 95% confidence interval, which is the bias (mean difference ± 1.96 SDs) in GFRs calculated with each method according to TPSM. Bias was calculated as follows: with n being the number of cases and dGFR the difference in GFR (other GFR − TPSM GFR).
with n being the number of cases and dGFR the difference in GFR (other GFR − TPSM GFR).
RESULTS
The patient data are presented in Table 1.
Patient Data
There was a statistically significant positive correlation between GFRs calculated using TPSM and those calculated using cystatin C (r = 0.904, P < 0.001) or β-trace protein (r = 0.725, P < 0.001). The correlation was stronger for cystatin C (73% vs. 90%). There was a significant negative correlation between GFRs calculated using TPSM and those calculated using cystatin C or serum β2-microglobulin but not those calculated using β-trace protein or creatinine: r = −0.797, P < 0.0001, for cystatin C; r = −0.762, P < 0.0001, for β2 microglobulin; r = −0.090, P = 0.417, for β-trace protein; r = −0.033, P = 0.769, for creatinine (Table 2).
Correlation Between TPSM GFR and GFRs Calculated with the Other Methods
Regarding the 3 KDOQI groups, only group 2 showed a significant correlation between TPSM and β-trace protein (r = 0.67, P < 0.0001), but Bland–Altman analysis did not reveal reliable agreement. Groups 2 and 3 showed a significant correlation between TPSM and cystatin C (r = 0.82, P < 0.0001, and r = 0.76, P < 0.0001, respectively), but again, Bland–Altman analysis did not reveal reliable agreement. The correlations calculated using cystatin C were similar in both groups, but those calculated using β-trace protein were lower than those calculated using cystatin C (Figs. 1 and 2; Tables 2 and 3).
Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and cystatin C GFR at confidence level of 95%. sisC = cystatin C.
Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and β-trace protein GFR at confidence level of 95%. TP2 = β-trace protein.
Agreement Between TPSM GFR and Cystatin C and β-Trace Protein GFRs
Regarding the analysis according to serum NAG level, the correlation between TPSM and cystatin C had a statistically significant r value of 0.957 in patients with a level below 6.1 and 0.887 in those with a level above 6.1. These correlation coefficients were significantly different (P < 0.0001), suggesting that cystatin C reflects GFR more accurately when urinary NAG is within normal limits (≤6.1)—that is, when the NAG level indicates that there is no tubular injury (Fig. 3). The correlation between TPSM and β-trace protein had an r value of 0.801 in patients with a level below 6.1 and 0.694 in those with a level above 6.1. These correlation coefficients were significantly different (P < 0.0001) (Table 4). Bland–Altman analysis was used to draw scatterplots of the difference between TPSM and cystatin C or β-trace protein in subjects categorized by urinary NAG level. The analysis revealed that there was no reliable agreement within a 95% confidence interval (Fig. 4).
(A) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and cystatin C GFR with urinary NAG ≤ 6.1 IU/L, at confidence level of 95%. (B) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and cystatin C GFR with urinary NAG > 6.1 IU/L, at confidence level of 95%. sisC = cystatin C.
(A) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and β-trace protein GFR with urinary NAG ≤ 6.1 IU/L, at confidence level of 95%. (B) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and β-trace protein GFR with urinary NAG > 6.1 IU/L, at confidence level of 95%. TP2 = β-trace protein.
Correlation Between TPSM GFR and β-Trace Protein and Cystatin C GFRs for the 2 Levels of NAG
Urinary β2 microglobulin is also a good marker for renal tubular dysfunction (28), with a reference range of 0.01–0.2 mg/L. In patients with β2 microglobulin below 0.2 mg/L, the r value was 0.849 (P < 0.01) for cystatin C. In patients with β2 microglobulin above 0.2 mg/L, it was 0.840 (P < 0.01). These values were significantly different (P < 0.0001). For β-trace protein, the respective values were 0.698 (P < 0.01) and 0.433 (P < 0.01), a difference that was also significant (P = 0.02) (Table 5). Bland–Altman analysis was used to draw scatterplots of the difference between TPSM and cystatin C or β-trace protein in subjects categorized by urinary β2-microglobulin level. Although there was a statistically significant correlation between 99mTc-DTPA and cystatin C or β-trace protein according to urinary β2-microglobulin (the highest value was r = 0.892 for cystatin C in patients with urinary β2-microglobulin ≤ 0.2), Bland–Altman analysis revealed that there was no reliable agreement within a 95% confidence interval (Figs. 5 and 6).
(A) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and cystatin C GFR with urinary β2-microglobulin ≤ 0.2 mg/L, at confidence level of 95%. (B) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and cystatin C GFR with urinary β2-microglobulin > 0.2 mg/L, at confidence level of 95%. sisC = cystatin C.
(A) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and β-trace protein GFR with urinary β2 microglobulin ≤ 0.2 mg/L, at confidence level of 95%. (B) Scatterplot drawn using Bland–Altman analysis showing difference between 99mTc-DTPA GFR and BTP GFR with urinary β2 microglobulin > 0.2 mg/L, at confidence level of 95%. TP2 = β-trace protein.
Correlation Between TPSM GFR and β-Trace Protein and Cystatin C GFRs for the 2 Levels of Urinary β2 Microglobulin
DISCUSSION
Several studies have shown that GFR measurement using 99mTc-DTPA or 51Cr-EDTA blood samples to show renal function gives results similar to those of continuous inulin infusion (23,24,29,30). Rehling et al. (8), in a study in which inulin was accepted as the gold standard, reported a 99mTc-DTPA-to-inulin clearance ratio of 0.97 and showed that 99mTc-DTPA clearance correlated fairly well with inulin clearance. They also found that the GFR measured using 99mTc-DTPA averaged only 3.5 mL/min higher than that measured using inulin clearance. In light of these findings, we accepted TPSM as the reference method and compared GFRs obtained using this method with those obtained using cystatin C and β-trace protein to investigate whether the latter two can be used reliably in clinical practice.
Various studies with different patient populations have investigated the role of cystatin C in measurement of GFR (31). However, the use of cystatin C in CRD patients is both limited and debated. Frank et al. (32) reported that in CRD patients, cystatin C gave higher but acceptable GFRs, whereas Maillard et al. (33) and Pöge et al. (34) suggested that cystatin C should be preferred to serum creatinine measurement.
Coll et al. (31) reported that serum cystatin C level had a higher sensitivity than serum creatinine (93.4% vs. 86.8%) for measurement of GFR. They stressed the importance of cystatin C for detecting early-stage renal failure in mild renal injury. Various studies (35) found that serum creatinine, cystatin C, and β2-microglobulin levels correlated closely with 51Cr-EDTA clearance (35,36). In addition, Oner et al. (37) compared serum creatinine, cystatin C, and β-trace protein in the determination of renal function in 89 transplant recipients and showed that serum cystatin C was a better marker than β-trace protein.
Rule et al. (38) observed a strong correlation (r2 = 0.853) between cystatin C and GFR, particularly in 204 patients with kidney disease. In that study, the correlation was better for cystatin C than for creatinine, but the authors pointed out that the correlation is affected by such factors as inflammation and immunosuppression and that cystatin C thus cannot be used as an ideal marker of GFR.
In our study, TPSM GFR was 31.9 ± 14.3 and cystatin C GFR was 46.8 ± 17.3. When the correlation between GFRs calculated using TPSM and using other methods was analyzed, the correlation was highest for cystatin C (r = 0.904, P < 0.001).
β-trace protein is a recently introduced alternative method of determining GFR. Various studies have shown that a reduction in GFR leads to a progressive elevation in β-trace protein. Some studies have reported that β-trace protein, serum creatinine, cystatin C, and β2 microglobulin are similarly accurate in detecting renal dysfunction. Kobata et al. (39) demonstrated that serum β-trace protein is an accurate marker for detection of early-stage renal failure in patients with type 2 diabetes mellitus. It was also reported that β-trace protein can be used as an alternative endogenous GFR marker in renal transplant recipients on steroid therapy (14). However, Oner et al. (37) reported that β-trace protein did not accurately reflect GFR in cases of renal transplantation.
Pöge et al. (14) studied the role of β-trace protein in reflecting GFR in 187 renal transplant recipients. The investigators accepted as the gold standard GFR calculated using 99mTc-DTPA and compared GFRs calculated using 3 separate formulas for β-trace protein with GFRs calculated using the reexpressed MDRD (Modification of Diet in Renal Disease) formula. GFRs calculated using β-trace protein were not significantly superior to those calculated using the reexpressed MDRD formula.
In our study, on the other hand, the correlation between TPSM and cystatin C was statistically significant (r = 0.904, P < 0.001) and better than that for β-trace protein (r = 0.725, P < 0.001). However, Bland–Altman analysis revealed no acceptable agreement between TPSM and either cystatin C or β-trace protein within a 95% confidence interval.
After grouping the subjects according to GFR, we found no statistically significant correlation between TPSM and cystatin C in group 1, whereas groups 2 (r = 0.821, P < 0.0001) and 3 (r = 0.764, P < 0.0001) did show a statistically significant correlation. Only group 2 (r = 0.671, P < 0.0001) showed a statistically significant correlation between TPSM and β-trace protein, albeit to a lower degree than cystatin C. Bland–Altman analysis in each of the 3 groups revealed no acceptable agreement between TPSM and cystatin C or β-trace protein within the 95% confidence interval. The correlation with TPSM was better for cystatin C than for β-trace protein in the 3 groups. However, neither method agreed sufficiently well with TPSM. In light of these results, we believe that cystatin C and β-trace protein are not suited for determination of renal function in CRD patients.
After grouping the subjects according to urinary NAG, we found the correlation between TPSM and cystatin C to have an r value of 0.957 in patients with normal NAG and 0.887 in patients with elevated NAG. These 2 coefficients were significantly different (P < 0.0001), suggesting that cystatin C reflects GFR more accurately when there is no tubular injury. Nevertheless, Bland–Altman analysis revealed no acceptable agreement between TPSM and cystatin C within the 95% confidence interval.
After grouping the subjects according to β2 microglobulin, we found a statistically significant correlation between TPSM and both cystatin C and β-trace protein in patients with a normal or high β2-microglobulin level, although Bland–Altman analysis showed no agreement within the 95% confidence interval. The correlation between the 2 GFRs was more significant for β2 microglobulin than for cystatin C.
CONCLUSION
We demonstrated that cystatin C and β-trace protein—although capable of suggesting impaired renal function—do not accurately measure GFR and therefore are not suitable markers of renal injury in CRD patients. The gold standard, TPSM, should be retained in CRD patients with or without renal tubular injury.
DISCLOSURE
This study was supported by the Akdeniz University Scientific Research Projects Unit. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jun. 25, 2015.
REFERENCES
- Received for publication February 9, 2015.
- Accepted for publication May 21, 2015.













