Visual Abstract
Abstract
177Lu-DOTATATE has gained wide clinical acceptance for the treatment of advanced gastroenteropancreatic neuroendocrine tumors; however, little is known regarding its accumulation in ascites. As such, clinical staff performing paracenteses shortly after a treatment dose may be concerned about their potential radiation exposure or the risk of contamination. Methods: In this report, therapeutic paracenteses were performed on a patient with metastatic intestinal carcinoid complicated by recurrent chylous ascites at various time intervals after a standard 7.4 GBq dose of 177Lu-DOTATATE. Samples of the fluid were analyzed in a scintillation counter to estimate the concentration of radioactivity. Results: The concentration of activity in the ascitic fluid obtained 3 d after an infusion was exceptionally low (175.3 ± 25.9 Bq/mL). Conclusion: Our findings suggest that paracenteses conducted as soon as 3 d after a standard dose of 177Lu-DOTATATE pose little to no risk in terms of radiation safety to staff performing the procedure.
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are generally considered an indolent class of neoplasms and tend to present in advanced stages of disease that respond poorly to conventional chemotherapies (1). Many GEP-NETs are initially diagnosed after nonspecific signs or symptoms related to tumor mass effect, invasion, or distant metastases present themselves. Several of the most common signs and symptoms include abdominal pain, weight loss, bloating, nausea, diarrhea, and jaundice. In rare incidences, patients with GEP-NETs may develop chylous ascites, which can occur either as a result of the obstruction of a lymph node by tumor invasion or fibrosis or by the impaired flow of lymph due to fibrosis of the lymphatic ducts in the surrounding tissues (2–5). Regardless of the cause, chylous ascites has been associated with more aggressive forms of the disease, as well as poorer outcomes, and often leads to additional challenges related to patient management (2,3).
Since its approval by the Food and Drug Administration in January 2018, 177Lu-DOTATATE has become a popular second-line treatment option in the management of advanced, well-differentiated somatostatin receptor–positive GEP-NETs that have progressed on conventional octreotide therapy (6,7). The typical therapeutic regimen includes 4 7.4-GBq doses of 177Lu-DOTATATE administered via infusion 8 wk apart. As is the case with most nuclear medicine procedures, clinical staff may be apprehensive about conducting interventional procedures shortly after the administration of 177Lu-DOTATATE because of fear of radiation exposure or contamination. This is particularly true of invasive procedures, such as paracenteses or thoracenteses, for which the potential of contamination is increased. Staff concern is complicated given that little is known about how much radioactive material accumulates within these fluids. Herein, we report on our experience performing paracenteses in a patient with metastatic ileocecal carcinoid complicated by recurrent chylous ascites after the standard 7.4-GBq treatment dose of 177Lu-DOTATATE as well as provide estimates of the concentration of radioactivity in the fluid. These data should serve to reassure clinical staff performing paracenteses of the extremely low radiation exposure during the procedure.
MATERIALS AND METHODS
Case History
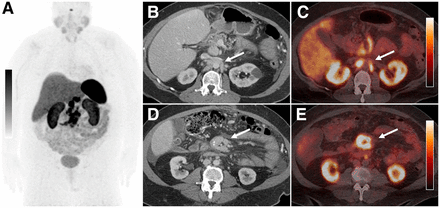
A 59 y old with a history of an ileocecal carcinoid tumor status after resection with metastatic somatostatin receptor–positive mesenteric and retroperitoneal adenopathy was referred to our nuclear medicine department for 177Lu-DOTATATE therapy (Fig. 1). The patient was previously treated with long-acting octreotide, and subsequently everolimus, both of which were terminated due to associated side effects and progression of disease. Several weeks before initiating therapy with 177Lu-DOTATATE, the patient began developing recurrent chylous ascites that required the therapeutic drainage of greater than 7 L of fluid every 3–4 d. A multidisciplinary team consisting of oncology, nuclear medicine, medical physics, and radiology staff performing the paracenteses discussed the case and agreed to proceed with the treatment as well as the as-needed therapeutic paracenteses, out of medical necessity. Although attempts were made to postpone the routine scheduled paracentesis immediately after the 177Lu-DOTATATE infusion, the patient required it after 3 d because of worsening abdominal discomfort. This occurrence prompted us to investigate the potential risk of radiation exposure to the clinical staff performing the procedure. Because of the limited number of subjects, our institutional review board deemed it unnecessary to submit this case for approval, and the requirement for informed consent was waived.
A 59 y old with metastatic ileocecal carcinoid tumor. (A) Anterior 68Ga-DOTATATE PET maximum-intensity-projection image showing intense somatostatin receptor avidity greater than that of the liver within a conglomerate of central abdominal lymph nodes. Axial contrast-enhanced CT images of abdomen with fused PET/CT images at same level illustrating examples of retroperitoneal (B and C) and central mesenteric lymph nodes (D and E) (arrows) that showed somatostatin receptor positivity on PET/CT. Scale bars = SUVmax = 14.
Infusion Protocol
177Lu-DOTATATE was administered using the standard infusion protocol (6,7). Briefly, the patient was premedicated with an antiemetic 30 min before initiating the infusion of an amino acid solution containing l-arginine and l-lysine. The amino acid infusion was started 30 min before, and continued during and 3 h after the 177Lu-DOTATATE infusion, and was infused at a rate of 250 mL/h. The 7.4-GBq dose of 177Lu-DOTATATE was infused over 20–30 min. The patient was instructed to void frequently over the course of their treatment to reduce the radiation dose to their kidneys and bladder. Before the patient left the nuclear medicine suite, 30 mg of subcutaneous long-acting octreotide was administered.
Paracentesis and Fluid Analysis
Fluid samples from paracenteses performed at various intervals after each of the first 3 doses of 177Lu-DOTATATE were collected and analyzed using a standard protocol. Samples were always collected during paracenteses performed 3 and 10 d after the treatment doses; however, samples could not always be obtained during paracenteses performed 7, 13, and 20 d after the treatment doses due to logistical factors.
Large-volume paracenteses were performed in the radiology department under ultrasound guidance. A 50-mL sample of the drained fluid was transported to the radiation safety department where it was interrogated with a NaI(Tl)-based survey meter (Exploranium miniSpec GR-130; Exploranium G.S. Limited). The scan revealed 2 peaks, at 208 and 113 keV, matching the expected γ-peaks of 177Lu (Table 1). Subsequently, 0.5-mL aliquots (n = 5 after the first treatment dose and n = 10 after the second and third treatment doses) were drawn and mixed with 7 mL of liquid scintillation cocktail fluid (Insta-fluor Plus; Perkin Elmer) in a standard 20-mL glass vial. A blank sample with no peritoneal fluid was also prepared. All samples were scanned in a liquid scintillation counter (Guardian1414; Perkin Elmer) using an open energy window (channel 5-1024).
Principal Emissions for 177Lu and Their Relative Intensities (Those > 1%)
RESULTS
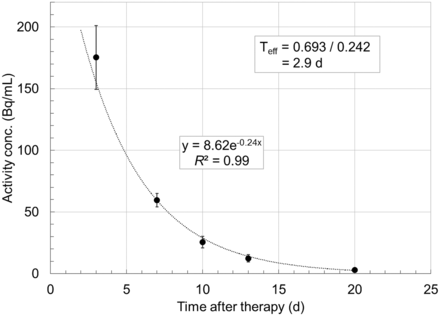
Table 2 shows the results of the liquid scintillation counter measurements of the fluid samples obtained at various intervals after the first 3 treatment doses. The mean activity concentration 3 d after a treatment dose was 175.3 ± 25.9 Bq/mL. The maximum activity within a total volume of drained fluid was estimated to be 1.42 MBq in 7.3 L, which was collected on day 3 after the first treatment dose. The residual activity fell rapidly after day 3, as can be seen from the subsequent measurements. Figure 2 is a graphical presentation of the data in Table 2. An exponential curve fit to the data yielded an effective half-life in the fluid of 2.9 d.
Mean Activity Concentration Based on Liquid Scintillation Measurements of Ascitic Fluid Samples Obtained at Various Intervals After Treatment Doses of 177Lu-DOTATATE
Plot of 177Lu activity concentration in ascitic fluid as function of time. Estimated effective half-life in ascitic fluid is 2.9 d.
DISCUSSION
For patients who must undergo paracentesis within a few days of a 177Lu-DOTATATE infusion, the issue of radiation safety to clinical staff can pose a tricky problem. However, the data presented here suggest that as soon as 3 d after an infusion, the concentration of activity in the peritoneal fluid is exceptionally low, on the order of approximately 175 Bq/mL. Given the low concentration of activity, the drained fluid can be safely disposed of via the usual biowaste channel. Moreover, the estimated activity implies that the associated radiation exposure to the clinical staff is negligible, and they should be able to safely conduct the procedure without the fear of high radiation exposure from the patient or contamination from the fluid. Normal precautions to avoid contamination, such as the use of gloves, gowns, and masks, should be sufficient for protection during the procedure.
The results of this case also suggest that the concentration of activity within the peritoneal fluid may follow a trend similar to that of the terminal blood activity of 177Lu-DOTATATE. Pharmacokinetic analyses have shown that the radiopharmaceutical rapidly clears from circulation with a mean effective half-life in blood of 0.31 ± 0.13 h (8,9). After most of the injected activity distributes to somatostatin receptor type 2–expressing cells or undergoes renal excretion, its terminal half-life in blood is estimated to be 71 ± 28 h (6). In our case, the concentration of activity within the ascites had an estimated effective half-life of 2.9 d, which is similar to its terminal half-life in blood. These findings suggest there is likely a pseudoequilibrium that exists between the concentration of radiopharmaceutical circulating in the blood pool and the concentration that accumulates in the ascites, which is skewed heavily toward the blood pool. Moreover, biodistribution studies have shown that less than 1 percentage of the injected activity remains in the blood pool after 24 h (8), and thus it is expected that minimal activity should be present within the ascites when a paracentesis is performed after 3 d.
Several limitations of this case should be noted. First, the results are based on our experience with a single patient, in part because chylous ascites is an incredibly rare complication of metastatic neuroendocrine tumors, specifically carcinoid. Second, due to the small sample size, we could not assess how the composition of peritoneal fluid or its rate of accumulation might affect the concentration of activity in the fluid. Last, we did not perform direct measurements of the radiation exposure to clinical staff performing the paracenteses. Rather, our inference that the radiation exposure is extremely low so as to be inconsequential is based on the measured radioactivity in the ascitic fluid. To this end, we have shown that minimal activity is present within the fluid even in a case of rapidly accumulating large-volume chylous ascites, which should be applicable to less severe cases of ascites.
CONCLUSION
Paracenteses conducted on patients as soon as 3 d after a standard dose of 177Lu-DOTATATE likely pose little to no risk in terms of radiation safety to the staff performing the procedure. The peritoneal fluid likely retains minimal levels of radioactivity and thus can be safely disposed in the usual stream of medical waste.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the risk of radiation exposure to staff from ascitic fluid collected during large-volume paracenteses in a patient who has recently been treated with 177Lu-DOTATATE?
PERTINENT FINDINGS: The results of this single case suggest that the concentration of activity in the ascites as soon as 3 d after a standard dose of 177Lu-DOTATATE is likely negligible at approximately 175 Bq/mL.
IMPLICATIONS FOR PATIENT CARE: Clinical staff performing paracenteses in a patient receiving 177Lu-DOTATATE therapy should feel comfortable knowing that the potential risk of radiation exposure and contamination is likely very low.
Footnotes
Published online Dec. 21, 2021.
REFERENCES
- Received for publication June 30, 2021.
- Accepted for publication November 30, 2021.