Visual Abstract
Abstract
The number of radioligand therapy applications for metastatic castration-resistant prostate cancer has been continuously rising in most nuclear medicine departments in Iran, but to our knowledge, no one has studied the dose to staff who perform treatment procedures. The current study aimed to determine the external radiation dose received by staff who, using or not using a lead shield, treat patients with 177Lu-prostate-specific membrane antigen therapy. Methods: This study used a personal thermoluminescent digital survey meter to measure dose rates to staff at various distances from patients and determined the average time spent by staff at these distances. The deep-dose equivalent to staff was obtained. Results: The measured deep-dose equivalent to staff per patient was within the range of 1.8–5.2 mSv using a 2-mm lead shield and 3.3–8.1 mSv not using the shield. The shield markedly reduced the external dose to staff. Conclusion: The skill and accuracy of staff, and the speed with which they act, can directly affect their received dose.
Recently, radioligand therapy targeted at the prostate-specific membrane antigen (PSMA) was introduced, and such therapy with 177Lu-PSMA-DKFZ-617 has shown promise for castration-resistant prostate cancer. The physical half-life of 177Lu is estimated at 6.73 d. 177Lu emits 2 types of radiation, namely β-rays (maximum energy, 0.498 MeV) and γ-rays (113 keV with 6% abundance and 208 keV with 11% abundance) (1–3). These γ-rays allow scintigraphy and subsequent dosimetry to be performed with the same therapeutic compounds. Because of the γ-rays of 177Lu, radiation protection can become an issue (4).
The aim of radionuclide therapy is to deliver an effective absorbed dose to tumor cells while protecting critical organs from an excessive radiation dose. Meanwhile, unnecessary radiation doses to family members, the medical team, and the general public must be avoided. In particular, nuclear medicine technologists come into close proximity to radiation sources when targeted therapy such as 177Lu-PSMA-DKFZ-617 is used, receiving radiation doses while preparing and administering the radioligand, positioning the treated patient on the scanner bed, controlling the patient during data acquisition, transferring the patient from the bed, and escorting the patient to the department (5). Thus, nuclear medicine societies have introduced several protective recommendations for targeted therapy, and various reports on methods of reducing the dose received by patients and staff have been published in some national and international journals (6).
Many investigators have measured the average external dose rates to staff, using pocket electronic and thermoluminescent dosimeters to record the total dose per study (7,8). There are two ways to directly determine the external radiation dose to staff per procedure: the first is based on accurate measurement of the dose rate at set distances from the patient and less accurate evaluation of the time spent by the operator at these distances, and the second is based on direct reading of an electronic dosimeter used by the staff during the procedure. The first tactic is a rough approximation of dose rate measurements but is more general and directly compares dose rates between different sets of published data (9).
The primary aim of this study was to determine the mean external dose to staff administering 177Lu-PSMA-DKFZ-617 therapy while using or not using a lead shield and while at different distances from the patient. A secondary aim was to determine the annual dose to staff administering this therapy.
MATERIALS AND METHODS
The study was authorized by the hospital ethics committee and was performed in accordance with the Declaration of Helsinki. All patients gave written informed consent. The inclusion criteria were an age of more than 55 y, the presence of metastatic castration-resistant prostate cancer, and treatment with 177Lu-PSMA-DKFZ-617. In total, 45 patients were enrolled from March 2019 to March 2020 (mean age, 66.2 y; range, 55–80 y) and were admitted to the Nuclear Medicine Department of Shohadaye Tajrish Hospital in Tehran, Iran. Demographic information on the staff is presented in Table 1.
Demographic Information on Staff
Four patients were treated on each therapy day in a 4-bed isolation room in the hospital’s day-procedure unit. The beds were located in the 4 corners of the room, which had an area of about 30 m2 and was shielded with lead (1.6 cm thick and 2 m high in the walls; 0.8 cm thick in the door) so that the patients could be isolated after the administration. The distance between beds was 2 m, and a mobile lead shield (2 mm thick) was placed between beds. The injection was prepared in a separate dedicated room. All patients were separately measured for dose rate in the lead-shielded room at specified intervals.
The study was performed using a digital survey meter (FH 40G-L10; Thermo Fisher), which was calibrated by a secondary standard dosimetry laboratory. This type of personal thermoluminescent dosimeter was chosen because it is capable of measuring photons in the range of 10 nSv/h–100 mSv/h and has a range of energy response of 30 keV–4.4 MeV; the dosimeters were dedicated to the 177Lu-PSMA-DKFZ-617 therapy procedures.
The patients were treated with a mean of 5.5 ± 1.1 GBq (range, 3.7–7.4 GBq) of 177Lu-PSMA-DKFZ-617. The dose rate at chest level was then measured at distances of 0, 0.25, 0.5, 1, and 2 m from the patients (10), once with and once without a 2-mm lead shield, after 0, 1, 2, 3, 4, 5, 6, 18, 24, and 36 h. The staff also recorded the mean time spent at each distance. Doses were measured as μSv/h and were converted to μSv/GBq⋅h according to the amount of radiopharmaceutical injected. Time (seconds) and relative dose rates were multiplied by each other. Finally, the mean (±SD) external doses to staff were calculated.
We routinely administer 177Lu-PSMA-DKFZ-617 treatment on an outpatient basis. The dose limit recommended by European guidelines for the discharge of patients after 131I therapy and by African guidelines on 177Lu-PSMA-DKFZ-617 was set as the basis for discharge (20 μSv/h within 1 m) (11–15). From Equation 1, one can estimate the cumulative dose, E, to a caregiver standing a specified distance away from the patient for an unlimited time (t), assuming that only physical decay occurs. We assumed a distance, D0, of 1 m and set an initial dose rate reading of 20 μSv/h at this distance. The half-life, t1/2, of 177Lu is 6.7 d. The calculation found E to be 4.6 mSv (16):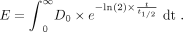 Eq. 1
Eq. 1
Data processing, data fitting, and the statistical analysis were performed using Excel (Microsoft Office Professional Plus, version 2013) and SPSS (version 16.0, IBM Corp.). The Kolmogorov–Smirnov method was used to investigate the normal distribution of data. A P value of 0.05 or less was assumed to indicate statistical significance. Data are presented as the mean and SD unless stated otherwise.
RESULTS
The mean dose rates at various distances and time intervals are presented in Table 2. The dose rate gradually decreased as activity was excreted from the body. Differences in injectable activity, tumor uptake, and renal function had a great impact on the rate of clearance. Because most patients did not start to urinate until about 1 h after infusion, the initial readings were the highest: 47.5 ± 2.0 μSv/(h⋅GBq) (range, 40.0–58.0 μSv/[h⋅GBq]) at 0.25 m, 21.5 ± 1.2 μSv/(h⋅GBq) (range, 18.5–24.5 μSv/[h⋅GBq]) at 0.5 m, and 7.1 ± 0.3 μSv/(h⋅GBq) (range, 5.5–8.3 μSv/[h⋅GBq]) at 1 m. The dose rate 1 m from the patient decreased exponentially with time after infusion. The average dose rate at this distance at 4–5 h was considered safe, as it was below the release limit required by our department (20 μSv/h).
Mean Dose Rates (μSv/[h⋅GBq]) and Related SDs at Various Distances from Patient and Various Times
Table 3 shows the per-patient dose to staff while using or not using the lead shield, and Table 4 shows the estimated mean annual dose to staff while using or not using the lead shield. The mean annual dose was calculated both for patients included in the study and for patients excluded and was determined using the data in Table 3 and the annual numbers of cases in the nuclear medicine laboratory. Table 5 shows the mean annual dose to staff as measured using the personal thermoluminescent dosimeters. The calculations are based on the number of treatment sessions in a year, with the assumption that nuclear medicine staff participated in all such sessions.
Total Dose to Staff per Patient
Estimated Mean Annual Dose to Staff With and Without Shielding
Mean Annual Dose to Staff as Measured with Thermoluminescent Dosimeters
Annual mean doses differed among staff in different job positions, with nurses receiving the highest minimum dose, at 3.8 mSv (Table 5), and a dose of 2.3 mSv (Table 4) while shielded. Technologists in charge of the injection and in charge of imaging received doses of 2.2 and 1.5 mSv, respectively, while shielded and 3.4 and 2.6 mSv, respectively, while not shielded (Table 4). Physicians and physicists received the lowest doses: 1.0 and 1.2 mSv, respectively, while shielded and 1.8 and 2.1 mSv, respectively, while not shielded (Table 4).
DISCUSSION
177Lu-PSMA-DKFZ-617 therapy of castration-resistant prostate cancer has been practiced at a few specialized centers around the world. Essential criteria for incorporating any new cancer therapy, including targeted therapy, are safety, efficacy, regularity, practicality, and affordability (7,8). If such therapy requires an extended stay in the hospital, patients may have extra costs to bear and face the possibility of acquiring a nosocomial infection. Also, patients may experience emotional disturbance due to the isolation such therapy requires. Our findings demonstrated that 177Lu-PSMA-DKFZ-617 is safe to apply as an outpatient protocol, since the external dose rate decreases below the 20 μSv/h threshold after approximately 4–5 h.
In a study by Demir et al. (7), patients could be discharged from the hospital when the dose rate fell below the determined threshold of 30 μSv/h after approximately 4–5 h. A similar study was performed by Calais et al. (17); patients reached the 1-m release limit of 25 μSv/h at a mean of 2.3 h, and all were released within 6 h. Differences in results among various studies may be due to differences in injected activity, biologic uptake, and radiopharmaceutical clearance.
In our study, the highest dose was received by nurses (without shielding, 8.1 μSv per patient), who routinely entered the isolation room at the beginning of infusion to meet the patients’ needs and observe them. The 177Lu-PSMA-DKFZ-617 therapy was scheduled for the same time each day, with the same nurse generally being present. Our department performs around 300 sessions of 177Lu-PSMA-DKFZ-617 therapy per year (45 patients treated 3–6 times at an interval of 8–12 wk).
In comparison to physicists and physicians, technologists in charge of injection received a high radiation dose (7.6 μSv per patient), as predicted, because they spend long hours preparing the 177Lu-PSMA-DKFZ-617 activity and stay close to the bedside during the infusion. Technologists in charge of imaging also received a considerable total dose (4 mSv per patient), because they accompany patients to the scintigraphy room, position them on the bed, and thus also spend significant time near them. Because technologists in charge of injection received a higher dose than those in charge of imaging, rotation of these two type of duties is recommended. Unlike nurses and technologists, physicians had a confined role during the therapy; their role in medical supervision required only sporadic attendance in the treatment room, resulting in a total dose of 3.3 μSv per patient. Lastly, physicists received a relatively low dose of 3.5 μSv per patient, resulting from their entering the isolation room to measure the dose rate.
Generally, our results were close to those of Demir et al. (7), who showed that the mean radiation doses to nurses and radiopharmacists were 6.0 and 4.0 μSv/patient, respectively, whereas physicists and physicians received 2.0 μSv/patient. That work analyzed the dose rate for 23 patients treated with 7,400 MBq of 177Lu-PSMA-DKFZ-617, and the total dose to the medical team was estimated by an electronic personal dosimeter. The estimated values from the study of Demir et al. are presented in Table 6 for comparison to our study. Differences in the results may be due to differences in experience, skill, time between examination and injection, and promptness of staff.
Comparison of Mean Dose (μSv per patient) from Current Study and from Another International Study
Some treatment centers may choose to hospitalize patients to monitor their condition or facilitate further medical examinations. If 4 patients were treated on each therapy day in the 4-bed isolation room, the nurse, who spent up to 4 h attending the 4 patients after infusion, received a mean dose of 26–53 μSv. This wide range reflects differences in nursing requirements, tumor burden in each patient group, and behavior of the individual nurse. Although patient privacy may be somewhat compromised in this situation, the ability of both patients and their caregivers (usually a family member or friend) to talk to fellow patients, share their individual experiences, and gain mutual support is, in itself, a valuable therapy for this rare disease, for which authoritative, firsthand patient information is relatively scarce.
Although no measured radiation dose to medical staff exceeded the allowed limit (20 mSv/y), it is recommended that a protocol be proposed to minimize staff exposure. This protocol would include improving work procedures, minimizing close contact with patients, and using equipment and shielding when contact is unavoidable. Table 3 indicates that a 2-mm lead shield decreased the dose to physicists, physicians, nurses, and nuclear medicine technologists significantly—by approximately 2 times. Tables 3 and 4 show that even without a rotation of the workforce, and even with a significant increase in the number of patients, the annual dose to individual staff would not reach the annual limit (20 mSv/y) defined by the International Commission on Radiological Protection. Annual doses as indicated by thermoluminescent dosimeters agreed with the estimated mean annual doses, except for technologists in charge of injection. The lack of agreement regarding the injecting technologists may have occurred because, in the same shift, these staff administered both therapeutic 177Lu-PSMA-DKFZ-617 and diagnostic radiopharmaceuticals. The doses may therefore have appeared lower than they really were.
Various studies have determined the dose reductions to nuclear medicine staff when lead shields and aprons are worn (18–20). He et al. (21) studied the effect of lead aprons on reducing the dose from 57Co, 33Ba, 137Cs, 99mTc, and 131I radionuclides and found that the effect was greatest for radiopharmaceuticals that emit γ-rays of less than 140 keV. Furthermore, Bayram et al. (10) showed that a 2-mm lead shield could reduce the external radiation dose to staff performing various diagnostic tests. If a shield thicker than 2 mm were to be used, the dose could be lowered even further. We emphasize that regardless of job position, staff should consider the use of protective equipment. Additionally, reducing the exposure time and increasing the distance from the radiation source are advisable when working with positron nuclides and other high-energy γ-ray sources.
One limitation of this study was the low number of patients, and another is that we did not use lead shielding of varying thicknesses (<2 mm or >2 mm). In addition, the sensitivity of measurement and imaging devices decreases over time; therefore, a larger quantity of radioactive material must be administered to obtain sufficient counts for a quality image. Because administering a larger quantity to patients also increases the dose to staff, the devices must be subjected to a regular quality control program. Provided that such safety precautions are undertaken, our data showed that 177Lu-PSMA-DKFZ-617 therapy for prostate cancer is safe and tolerable and that external radiation doses to medical staff were within the allowable limits.
CONCLUSION
A 2-mm lead barrier reduced the dose to staff for the therapeutic procedures performed in this study. Thus, it is recommended that this protective device be used at all treatment stages. No measured radiation doses to staff exceeded the annual limit of 20 mSv/y.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
This article was extracted from the thesis of Elahe Mahmoudi, School of Medicine, Sahid Beheshti University of Medical Sciences, Tehran, Iran (project 410, 9,139).
KEY POINTS
QUESTION: What is the radiation dose to staff from administering radioligand therapy for metastatic castration-resistant prostate cancer?
PERTINENT FINDINGS: The amount of radiation dose to staff from treatment of patients with 177Lu-PSMA-DKFZ-617 was within the allowable range. The results were statistically significant.
IMPLICATIONS FOR PATIENT CARE: Lead protection can reduce the radiation dose to staff. This finding underscores the need for staff to consider use of shielding.
Footnotes
Published online Dec. 6, 2021.
REFERENCES
- Received for publication August 29, 2021.
- Accepted for publication October 22, 2021.