Abstract
The treatment for differentiated thyroid cancer consists of thyroidectomy followed by radioactive iodine therapy (RIT), in which the patient remains in isolation until the dose rate of the radioactive iodine reduces to a certain limit. The present research intends to estimate the length of stay of patients who undergo RIT, with radiometry analysis performed throughout the patient’s admission. Methods: Information such as age, sex, weight, height, prescribed activity, volume of liquids ingested, and the use of recombinant human thyrotropin was gathered from 204 patients with differentiated thyroid cancer who underwent RIT. During the admission, the dose rate was periodically measured. The data served as variables for a multiple regression, in which the coefficients (the term coefficient in this paper is related to the multiplicative factor of each considered variable used in Eqs. 1–4) and significance of each were verified as a function of the dose rate. Results: The results showed that length of stay, administered activity, volume of liquids ingested, use of recombinant human thyrotropin, and patient weight impacted significantly on the dose rate. The average effective half-life of the 131I, considering all patients, was 12.61 ± 3.28 h, and the average time for their radiologic release was 15.23 ± 5.50 h. On the basis of the results, it was possible to develop a tool to estimate a patient’s length of stay and effective half-life. Conclusion: The results can contribute to optimization of the radiologic protection of patients who undergo RIT, as well as allow better logistics regarding their admission, which can lead to more appropriate accommodation for the patient and a better use of resources.
Differentiated thyroid cancer (DTC) represents about 90% of thyroid cancers (1,2), and depending on the clinical case, the initial treatment consists of partial or complete thyroidectomy (1). The most common postoperative treatment is radioactive iodine therapy (RIT) with 131I (3,4). RIT can also be useful to suppress normal thyroid tissue and effectively eradicate the serum levels of thyroglobulin, increasing its specificity as a tumor marker and still improving the sensibility of 131I in cases of disease resurgence (5).
According to the International Atomic Energy Agency and the International Commission on Radiological Protection (6), the therapeutic use of radioisotopes carries a potential risk of radiation exposure for family and individuals close to the patient, as well as the environment and the staff at the nuclear medicine (NM) facility. Therefore, this practice should follow all established guidelines to ensure safe release of the patient (7).
The International Commission on Radiological Protection and the International Atomic Energy Agency do not determine the requirement for hospitalization in RIT, emphasizing the importance of criteria that include administered activity values, treatment-related dose potentials, the interests and socioeconomic status of the patient and family members, and even the conditions of the local health system (8–11). In Brazil, hospitalization of patients receiving an activity of 1,850 MBq (50 mCi) or higher is mandatory (12). This requirement may vary by country but meets the need to protect family members, the public, and even the environment from possible exposure or radioactive contamination (13). This condition demands an adequate physical structure to accommodate the patient and ensure that radiation protection standards are going to be followed until it is safe to discharge the patient.
The time it takes for radioactive material to be excreted from an organism is directly related to physical, biologic, and effective radioisotope half-lives (6). The length of stay for a patient receiving RIT may vary according to the prescribed and administered activities and the patients themselves, because of their own unique metabolism. For better control and a more appropriate length of stay, it is necessary to take regular readings of the dose rate, or even exposure rate, from the patient, verifying exactly when it hits the threshold of the legislation in force (14).
Exposure rate measurements can make huge contributions to radiologic protection regarding not only the patients but also the staff who deal directly with them. Thus, considering that the inpatient becomes a radioactive source, that there are basic precepts of radiologic protection in NM to be followed (especially those about RIT), and that there is a need for suitable and optimized therapeutic planning, this study aimed to investigate the length of stay of patients who undergo RIT by analysis of the radiometry.
MATERIALS AND METHODS
Data from 204 patients with DTC who underwent RIT were collected. The choice of using data from patients with DTC was related to its prevalence among thyroid cancers—about 90% (1,2). The considered administered activities were 3,700 MBq (100 mCi), 5,550 MBq (150 mCi), 7,400 MBq (200 mCi), and 9,250 MBq (250 mCi), according to the routines practiced in the participating NM facility. Each patient was given a unique identification number, and no changes were made to the service routine where the study was performed. All procedures regarding admission of patients to the facility, as well as 131I administration, were executed by the facility’s professionals. No orientations other than what the NM service provided to the patients were given. Additionally, all ethical aspects regarding information obtained through research development were respected. The research was submitted to a Brazilian ethics committee for evaluation, receiving approval according to report 2.650.168. Written informed consent was obtained from each patient before participation in the study. Patient could withdraw at any time, and no incentives were offered.
The data used in this research were collected from 2 different sources. From the patient’s medical records, we collected sex, date of birth, height and weight (for body mass index [BMI]), the activity values (prescribed and administered to the patient), and whether recombinant human thyrotropin (rhTSH) was used. Radiometric information (day, time, and dose rate in μSv/h) and the hydric volume ingested over the admission period were collected periodically during the patient’s stay. All data were recorded in a digital spreadsheet.
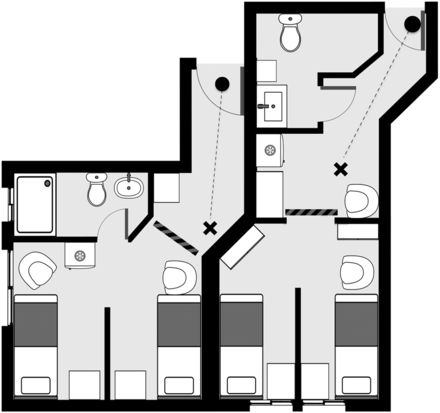
The first measurement was performed when the patient was admitted, immediately after radioactive iodine administration (0 h). The measurements then took place every 2 h, and the difference was registered in minutes, in relation to the initial time. The measurements themselves were performed in the therapeutic room while the patient remained in orthostasis in front of the researcher 2 m away, as defined by regulation NN 3.05, from the Brazilian National Commission of Nuclear Energy. The patient’s and the researcher’s positions in both therapeutic rooms are illustrated in Figure 1. If 2 patients were sharing the same room, a movable lead screen to isolate the subjects was used, reducing possible influence on measurements, and the other patient was asked to remain seated on the bed.
Patient’s and researcher’s positions (cross and dot, respectively) in both therapeutic rooms. Lead screen used to shield radiation is represented as striped line and was positioned before measurement of dose rate, reducing influence of other patients.
To ensure that distance was being maintained, the researcher pointed a laser measuring device (model GLM 20; Bosch) with an accuracy of ±3 mm toward the patient’s xiphoid process. The researcher then approached or moved away from the patient until there was exactly 2 m of distance between them. Afterward, a digital Geiger–Müller counter (model RadEye G20-10; Thermo Fisher Scientific), with a reading sensibility between 0.01 and 2.00 mSv/h and an energy range between 17 keV and 3 MeV, properly calibrated, was positioned at the very limit of the measuring distance.
The volume of liquid ingested by patients was recorded at each measurement. There was a large range of beverages in the facility to which patients had access, such as bottled water, orange juice, coconut water, coffee, and tea. The total volume of liquids ingested by each patient was based on the type of each drink multiplied by the known volume.
The subjects were also classified according to age: patients younger than 20 y were classified as teenagers, patients 20–39 y old as young adults, patients 40–64 y old as middle-aged adults, and patients older than 65 y as elderly.
To determine whether dose rate measurements had any influence on patients who were sharing a therapeutic room, the average measurements were compared between patients who were admitted alone to the facility and patients who shared a therapeutic room.
A multiple-regression model was created to analyze the statistical data of all gathered factors. Thus, all factors that mathematically had some sort of influence over the length of stay of the subject were identified and defined by their statistical importance and by the extent of that influence, using the coefficients of the patients. (The term coefficient in this paper relates to the multiplicative factor of each considered variable used in Eqs. 1–4.)
Two methods were used to evaluate the statistical importance of each coefficient. First, the P value coefficient was determined: coefficients that were considered statistically important were those for which the P value was no more than 0.05. Later, to check the analysis, a t test was performed to test the importance of the coefficient’s regression. As a supplement to the importance analysis, the Cohen f2 was used to quantify the potential importance of interference in a factor.
RESULTS
In total, 204 patients who underwent RIT were included. Table 1 presents the effective half-lives and the total lengths of stay until the dose rate reached the 30 μSv/h limit, organized by research categories. The coefficient of each considered variable and its SD, as well as the results of t tests (T) and their significances (P), are presented in Figure 2.
Effective Half-Life and Total Length of Stay According to Different Variables
Variables considered and their influence on dose rate. Coefficients and SDs are presented, as well as results of t test and significance.
Time, activity, volume of liquids ingested, weight, and use of rhTSH were all statistically relevant to the dose rate for all patients considered. The coefficient of determination (R2) was 0.815, indicating that the independent variables explain 81.5% of the dose rate. The Cohen f2 demonstrated that weight (f2 = 0.061), volume of liquids ingested (f2 = 0.059), and use of rhTSH (f2 = 0.091) had a small effect size, whereas time (f2 = 0.720) and prescribed activity (f2 = 0.564) had a large effect.
After calculating the coefficients of each variable (mean value for all patients), we could write Equation 1, which demonstrates the mathematic connection between the variables and the dose rate: Eq.1where D is the dose rate (μSv/h), h is the patient’s height (m), a is the patient’s age (y), m is the patient’s weight (kg), L is the volume of liquids ingested (L), r is use of rhTSH, A is the prescribed activity (MBq), s is the patient’s sex, and t is the time (h). Rewriting Equation 1, we could isolate time and have D(t), as shown by Equation 2:
Eq.1where D is the dose rate (μSv/h), h is the patient’s height (m), a is the patient’s age (y), m is the patient’s weight (kg), L is the volume of liquids ingested (L), r is use of rhTSH, A is the prescribed activity (MBq), s is the patient’s sex, and t is the time (h). Rewriting Equation 1, we could isolate time and have D(t), as shown by Equation 2: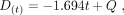 Eq. 2where Q represents all variables and coefficients, except t.
Eq. 2where Q represents all variables and coefficients, except t.
Thus, using Equation 2, one can estimate the time necessary for the dose rate to reach 50% of the initial level (0.5 D(0)), representing the effective half-life (Teff) of the radioactive iodine, as shown by Equation 3: Eq. 3
Eq. 3
In addition, it is possible to estimate the total hospitalization time (Ttotal) until the dose rate reaches 30 μSv/h, as shown in Equation 4: Eq. 4
Eq. 4
Regarding the subgroups of patient characteristics, the results for the coefficients and their respective significances were altered, as shown in Table 2.
Coefficients and Their Significance According to Different Variables
On the basis of the equations previously described, it was possible to develop a widget that calculates patient length of stay and effective half-life of the radioactive iodine. The result depends on the dose rate at the very beginning of the admission. If estimation of the total length of stay before admission is desired (without any measured dose rate), the calculator estimates the initial dose rate using the prescribed activity, as shown in Equation 5: Eq. 5where D represents the calculated dose rate (μSv/h), A is the prescribed activity (MBq), and d is the distance between the gauge and the patient (m). The widget calculator is freely available online (http://android.florianopolis.ifsc.edu.br/rit.php).
Eq. 5where D represents the calculated dose rate (μSv/h), A is the prescribed activity (MBq), and d is the distance between the gauge and the patient (m). The widget calculator is freely available online (http://android.florianopolis.ifsc.edu.br/rit.php).
DISCUSSION
Administered activity, volume of liquids ingested, use of rhTSH, and patient weight significantly affected dose rate reduction, whereas age and sex did not. The average effective half-life of the 131I was 12.61 ± 3.28 h, and the average time for radiologic release of the patients was 15.23 ± 5.50 h. On the basis of the results and the data collected, it was possible to develop a tool to estimate each patient’s length of stay and effective half-life.
All patients underwent total thyroidectomy because of DTC. DTC occurred predominantly in women, with a ratio of 3:1 for women to men, as agrees with other studies (14–18). DTC occurred most often in middle-aged patients, as corroborates other studies (18), followed by young adults.
Several studies have sought to relate patient BMI to various NM topics, from assessment of the risk of developing thyroid cancer according to BMI (19,20) to the influence of this parameter in image processing (21). The connection between thyroid cancer incidence and BMI stems from the fact that obesity is a widely known risk factor for thyroid cancer; thus, theoretically, the higher the BMI, the higher the incidence of the disease. However, nearly half of this study’s patients were overweight, followed by patients with a normal body weight and then obese patients. The group of underweight patients was not representative in this research.
The prescribed activity for the treatment was based exclusively on medical criteria and ranged from 3,700 MBq (100 mCi) to 9,250 MBq (250 mCi), with 3,700 MBq (100 mCi) being the most common, as corroborates other studies (1,22).
Regarding differences between prescribed and administered activities, there was little variation, with a SD of at most ±60.68 MBq (1.64 mCi). The same deviation was found in other studies (14) and is consistent with possible differences in activities prepared in clinical practice.
When calculating activity from dose rate, we observed a difference. Conversion of dose rate to activity can introduce errors, since the calculation is based on a point source (14). Other remaining factors may also influence dose rate measurement, such as background radiation. If the radiation in the environment (6,23) is higher than usual, the measurement results can be affected. In this research, room contamination can be disregarded because the room was completely cleaned and decontaminated before admission of a new patient.
By comparing the average dose rate measurements in patients who were given therapeutic activities of 3,700 MBq (100 mCi) and shared the room with another patient, we saw a slight increase in average dose rate as the activity administered to the other patient increased. For patients who received 3,700 MBq (100 mCi) and shared a room with another patient who received the same administered activity, the average dose rate was 59.27 μSv/h. For those who shared a room with a patient who received 5,550 MBq (150 mCi), the average dose rate was 60.51 μSv/h. For those who were with a patient who received 7,400 MBq (200 mCi), the average dose rate was 64.80 μSv/h. Considering these scenarios, the presence of another patient emitting radiation in the same environment may have some influence over the dose rate measurement, although there are other factors to be considered, such as BMIs and the distinct activity values. In this case, if a higher background radiation increases the dose rate measured for one patient, its effect will only be to delay release of the patient.
The use of shields and better use of the geometry of the environment may help lower this influence. When average dose rate measurements were compared between patients who were admitted individually and patients who shared a room, no significant difference was found; the average dose rate of individually admitted patients was 61.10 μSv/h.
The effective half-life we found matches the findings of other studies (14,24). However, when the sex of the patient was considered, the effective half-life of female patients was slightly shorter than that of male patients, going against the findings of other researchers (24). Such results may be related to a possible more aggressive disease condition among this group, leading to a higher uptake of radioactive iodine in the body (25). In addition, the time to reach the dose rate limit was about 3 h longer in men than in women.
Elderly patients had effective half-lives almost 1 h higher than in the other age groups. This result may have to do with metabolic slowdown and changes in kidney function as a person ages. Younger patients tend to have better kidney function (25), which may influence the decrease in effective half-life. However, patient age does not directly correlate with length of stay, which greatly depends on the clinical condition of the patient (25). Thus, it is important to assess the characteristics, clinical condition, and entire health history of the patient.
Obese patients generally had higher effective half-lives than the other weight groups, as can be related to metabolic aspects. However, obese patients also had a lower average length of stay than normal-weight or overweight patients. By receiving the 131I, the patient as a whole becomes the source, given the biodistribution of the element; the radiation is emitted throughout the body. An obese patient has a larger body than a normal-weight patient, influencing the amount of radiation detected. Still, the higher the body mass of the patient, the more will radiation be scattered because of its interaction with the body tissues (26).
The effective half-life found in this research varied in direct accordance with the prescribed activity, going from 12.11 ± 2.66 h for patients who received 3,700 MBq (100 mCi) to 13.77 ± 4.96 h for patients who received 7,400 MBq (200 mCi). Such results differ from other studies (14), which noted a decrease in effective half-life as administered activity increased. Such differences can be related to the condition of the patient or to the radioactive iodine uptake, since patients who are given higher activities usually have a tumor other than DTC, which has a low uptake of radioactive iodine.
Although the effective half-life largely depends on biologic factors related to the metabolism of radioactive iodine in the body, the physical characteristics of the nuclear disintegration indicate that the greater the administered activity, the greater the time required to reach a certain dose rate (6). The results showed that as activity became higher, hospitalization time increased up to 200%.
The use of rhTSH is another variable that influenced differences in length of stay and effective half-life. Patients who used the medication had effective half-lives about 2 h shorter than those who underwent hormone suspension. The same result was found when considering length of stay. The rhTSH group took, on average, 4 h less to reach the borderline dose-release rate then did the nondrug group, in line with other studies (24,27).
Likewise, the patients’ hydric intake seemed to influence effective half-life and, consequently, length of stay. Patients who ingested a smaller volume of fluids had a longer effective half-life than patients who ingested larger volumes. Hydric intake varied widely among patients, since it depended largely on their drinking characteristics. Adequate hydration may lead to dilution of radioactive iodine in the urine, which leads to reduced retention of the radioisotope in the urinary tract and contributes to decreased dose absorption in the bladder and adjacent tissues (28).
Exposure to ionizing radiation does not depend only on length of stay, as there are biologic and physiologic aspects that cannot be manipulated. However, optimizing the hospitalization process and encouraging patients and NM services to use good hygiene and safety practices can influence exposure time. Having a prior notion of how long the patient will be hospitalized can lead to optimization of hospitalization schedules, allowing attention to more patients (25) while reducing hospitalization costs regarding materials, supplies, or even personnel. Lower activities, such as 3,700 MBq (100 mCi), contributed to an average length of stay of 12 h until the dose rate reached 30 μSv/h, against the 35 h for higher activities, such as 9,250 MBq (250 mCi). Therefore the logistics for the hospitalization can also be improved, allowing the administrator to alternatively schedule patients to complete 24- or 48-h periods of room occupancy. Although discharge from RIT takes into consideration the patient’s clinical condition and not just radiometric criteria, it can assist in decisions about patient release and overall therapy planning.
In addition to the advantages of planning before treatment, the use of optimization tools can also be important during the hospitalization itself, serving as a patient feedback system. Thus, for example, patients with no kidney problems may be encouraged to ingest adequate fluids, aiming to accelerate excretion of the radioactive iodine (29).
Also, optimizing the hospitalization process directly benefits the patient’s well-being. Demystification of the hospitalization process and all issues related to ionizing radiation is important to create the most pleasant experience possible for a patient who arrives at the NM service having already endured the discovery of cancer and a surgical intervention. Moreover, the term nuclear is not well regarded by the overall population, in view of the nuclear attacks during World War II and nuclear accidents that have occurred around the world. NM suffers, to some extent, from the stigma left by such events (30,31), even though NM represents only 12% of the total dose received by an individual in the U.S. population in 2006 (23), considered in the context of other forms of exposure to ionizing radiation, natural or not.
A longer length of stay may have a negative psychologic effect on patients, since the hospitalization itself is already the result of a health problem (32,33). A shorter hospitalization and special attention from the team that assists the patient are fundamental to the comfort and the physical and mental well-being of the patient. Knowing the approximate length of stay can be comforting and help the patient make plans.
Establishing a standard effective half-life or determining how long a patient will actually be hospitalized is complex, especially when considering that procedure recommendations and guidelines generally consider the radioisotope’s physical half-life rather than its pharmacologic and biokinetic characteristics. However, closer control of the hospitalization process can result in higher-quality service, even impacting the overall quality of the treatment itself. As pointed out by the International Atomic Energy Agency (34), the future of all therapy is an individualized approach that respects the differences and interests of the patient, as well as of society as a whole.
CONCLUSION
Our results can contribute to decision making on how to efficiently accommodate DTC patients during RIT. Although the length of stay may vary among patients and among all involved variables, the calculator we have developed allows estimation of effective half-life and total length of stay, contributing to the patients’ well-being. The calculator is also useful for management of booking and patient load, optimizing scheduling of treatment while lowering costs related to long stays.
DISCLOSURE
This work was funded by the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC). No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Bionuclear Clinic and its multidisciplinary team for allowing the use of their data and actively assisting in the collection process.
Footnotes
Published online Oct. 5, 2020.
REFERENCES
- Received for publication May 11, 2020.
- Accepted for publication July 29, 2020.