Abstract
Cardiac involvement in sarcoidosis is associated with poor prognosis. 18F-FDG PET can detect the presence of cardiac sarcoidosis, assess disease activity, and serve as a means to monitor treatment response in patients with cardiac sarcoidosis.
Cardiac involvement occurs in 20%–30% of patients who had systemic sarcoidosis as shown in pathology examinations and is associated with poor prognosis (1,2). Although myocardial granulomas can be identified in almost 25%–79% of autopsy examinations, only 25% of all patients with sarcoidosis have clinical manifestations of cardiac involvement (1). In this report, 18F-FDG PET helped in diagnosing a patient with suspected cardiac sarcoidosis, classifying the disease activity, and monitoring the response to treatment.
CASE REPORT
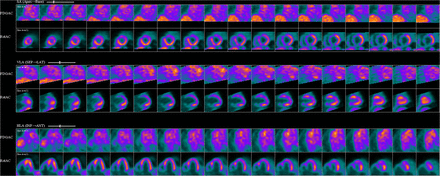
A 68-y-old man with a history of cutaneous sarcoidosis presented to the cardiology clinic with progressive dyspnea. Physical examination revealed +4-pitting edema. Electrocardiography showed complete heart block. The results of cardiac MR imaging suggested an infiltrative process. The patient was instructed to take a high-fat low-carbohydrate diet the day before imaging, without an additional overnight fast. With a baseline blood glucose level of 93 mg/dL, the patient was injected with 3.94 Bq (10.67 mCi) of 18F-FDG, and after a 1-h delay, multiple metabolic tomographic images of the myocardium were obtained. The 13N-ammonia perfusion images (Fig. 1, bottom) demonstrated defects in the mid to basal inferior wall, basal anterior walls, and anteroseptal and inferoseptal areas, as indicated by a correspondingly increased level of 18F-FDG uptake in these regions. This finding was consistent with active sarcoid infiltration of the myocardium. The calculated ejection fraction was 24%. The patient completed a 6-mo trial of high-dose steroids, after which a follow-up myocardial PET study (Fig. 2) demonstrated moderate improvement of the perfusion defects (whereas the 18F-FDG portion demonstrated only blood-pool activity without myocardial trapping). The ejection fraction improved to 37%. These findings indicated an excellent response to treatment of the existing inflammation, although clinical improvement depends on the extent of previous damage.
Perfusion images demonstrating defects in mid to basal inferior wall, basal anterior walls, and anteroseptal and inferoseptal areas, as indicated by correspondingly increased 18F-FDG uptake in these regions.
Improvement of perfusion defects as indicated by only blood-pool activity being seen on 18F-FDG images.
DISCUSSION
Cardiac sarcoidosis represents the cause of death in 13%–25% of fatal cases of sarcoidosis (1). A definitive diagnosis of cardiac sarcoidosis can be made by endomyocardial biopsy, with sensitivity being less than 20% (2). For a definitive diagnosis and management, all patients will likely benefit from an echocardiogram and either cardiac MR imaging or 18F-FDG PET (1). Cardiac MR imaging is usually performed as the initial test in patients with suspected cardiac sarcoidosis. Gadolinium-enhanced cardiac MR imaging with delayed imaging has the benefit of high sensitivity and spatial resolution without radiation exposure. If the MR results are negative, 18F-FDG PET can be avoided. If the MR results suggest cardiac involvement, 18F-FDG PET imaging may be performed to establish baseline disease activity, assess the need for initiation of medical therapy, and monitor response to treatment over time. In patients with contraindications to cardiac MR imaging, 18F-FDG PET can be used as the first-line imaging method (1). Usually, cardiac MR imaging shows scarring, and thus chronic disease, whereas 18F-FDG PET identifies active involvement. Typical radionuclide protocols for imaging cardiac sarcoidosis include 18F-FDG PET for imaging inflammation combined with SPECT or PET myocardial perfusion imaging (1).
The myocardial perfusion assessment can be performed with 99mTc, 201Tl, 13N-ammonia, or 82Rb-based radiotracers using standard protocols. Attenuation correction should be applied to SPECT myocardial perfusion imaging whenever possible. In the absence of coronary artery abnormalities, perfusion defects seen on PET scanning in a patient with sarcoidosis strongly suggest that the heart is involved (2). One way to describe the pattern in cardiac sarcoidosis on PET scans is to compare the degree of perfusion abnormality with the intensity of 18F-FDG uptake: normal (normal perfusion/normal 18F-FDG), early stage (mild perfusion defect/increased 18F-FDG), progressive stage (moderate perfusion defect/increased 18F-FDG), progressive myocardial impairment stage (severe perfusion defect/increased 18F-FDG), and fibrosis stage (severe perfusion defect/minimal or no 18F-FDG uptake) (1). We used this method, and the results were descriptive of the progressive myocardial impairment stage (Fig. 1). Our patient showed clinical improvement after 6 mo of treatment, and the PET findings resolved. 18F-FDG uptake was consistent with subsided inflammation, although clinical improvement (Fig. 2) is ultimately limited by the previous damage.
CONCLUSION
18F-FDG PET can detect the presence of cardiac sarcoidosis, assess disease activity, and monitor treatment response in patients.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Aug. 13, 2015.
- Received for publication April 6, 2015.
- Accepted for publication July 1, 2015.