Abstract
In patients with head and neck tumors, preoperative lymphoscintigraphy can be used to map lymphatic drainage patterns and identify sentinel lymph nodes. However, it is very difficult to determine the exact locations of head and neck sentinel nodes on preoperative lymphoscintigraphy without the use of anatomic landmarks. Lymph nodes in the head and neck are grouped into 7 regions, or levels, on the basis of anatomic landmarks. In patients undergoing standard lymphoscintigraphy, obtaining lateral marker images that show important anatomic landmarks can help with the localization of sentinel nodes. However, technical problems often render marker images of little or no use. Hybrid SPECT/CT lymphoscintigraphic imaging facilitates the localization of sentinel nodes by reliably showing the relationships between sentinel nodes and important anatomic structures. After reading this article, the reader should understand the lymph node level classification system for head and neck melanomas, be able to describe the technique used for the imaging of sentinel nodes in the head and neck region, and be able to demonstrate how SPECT/CT lymphoscintigraphic imaging can enable precise sentinel node localization and thus help to ensure minimal dissection.
Cutaneous malignant melanoma is a relatively common tumor. Early melanoma is highly curable, but once the disease becomes disseminated, it is nearly always fatal. Malignant melanoma should be suspected when any pigmented skin or mucosal surface lesion changes in color or size or begins to itch or bleed. In patients with a histopathologic diagnosis of cutaneous melanoma, the tumor must be staged to determine prognosis and treatment. Tumor thickness is the single most important prognostic factor. In the Breslow system, thickness is measured with an ocular micrometer to determine the maximum vertical thickness (<0.76 mm for stage I, 1.5–4.0 mm for stage II, and metastasis for stage III) (1–4). Melanoma is staged with the TNM system, in which T represents tumor thickness and ulceration, N represents spread to lymph nodes, and M represents metastasis to distant organs (5). Preoperative lymphoscintigraphy can contribute to nodal staging by revealing lymphatic drainage patterns and the locations of single or multiple sentinel lymph nodes at a specific ipsilateral, bilateral, or contralateral location relative to the primary tumor site. This information, in turn, helps the surgeon to minimize the size of the incision (6). If a sentinel node is positive for melanoma metastasis, then the disease is stage III, which has a 20% 10-y survival rate; the survival rates are 85% for stage I and 60% for stage II (7). The survival rate for stage III melanoma at diagnosis is approximately 10% (8). The estimated number of new cases of cutaneous melanoma in 2006 is 62,190, and the estimated number of deaths is 7,910 (9).
In patients with cutaneous melanomas of the head and neck, preoperative lymphoscintigraphy is challenging because of the complexity of the lymphatic drainage system in this region. The head and neck region contains a network of more than 350 lymph nodes in an exceptionally compressed area (Fig. 1) (6); therefore, melanoma of the head and neck is one of the most difficult tumors for which to predict the lymphatic drainage pathway. The intricacy of the head and neck lymphatic system translates to several technical challenges for preoperative lymphoscintigraphy. First, the intradermally injected isotope travels very fast in the head and neck region and may be taken up by multiple lymph nodes within a minute; therefore, it is critical to obtain dynamic images to capture the first node (sentinel node) to which the isotope travels. Second, even when a sentinel node is visualized, it can be difficult to differentiate sentinel from nonsentinel nodes when multiple nodes appear, because the nodes are too small to be distinguished from one another during planar imaging (10). The third, and most common, problem is masking of the sentinel node by the injected tracer because of the close proximity of the tumor site to the draining node. Most often, the sentinel node is overlooked because of the smear of the tracer overlying the draining node (10). The sentinel node is mostly obscured when the primary lesion site is located at the neck and ear area. The sentinel node may be obscured on planar images if the injection site near the original tumor in the neck overlies jugular or cervical lymphatic channels.
Illustration of complex lymphatic system of head and neck region.
LYMPH NODE LEVEL CLASSIFICATION
The cervical lymph nodes are divided into 7 regions, or levels (Fig. 2), according to the system proposed by Som et al. in 1999 (11). This method of grouping the nodes was designed to be easily and readily used with imaging-based modalities. The level I nodes are located at the chin level, superior to the mandibular angle, and are subdivided into IA and IB. The level IA nodes, the submental nodes, can vary in number from 2 to 8 and are located between the medial margins of the anterior bellies of the digastric muscles (12). The level IB nodes, the submandibular nodes, lie above the hyoid bone, between the digastric muscles, below the body of the mandible (13). The level II nodes, the upper jugular nodes, are also divided into 2 groups. The level IIA nodes are located at the mandibular angle above the hyoid bone and anterior to the sternocleidomastoid muscle, whereas the level IIB nodes are located above the hyoid bone and adjacent to the sternocleidomastoid muscle. The level III nodes, the middle jugular nodes, are located between the hyoid bone and the cricoid cartilage, adjacent to the sternocleidomastoid muscle. The level IV nodes, the lower jugular nodes, are located between the cricoid cartilage and the clavicle, adjacent to the sternocleidomastoid muscle. The level V nodes, the posterior triangle nodes, are divided into 2 groups. The level VA nodes are located above the cricoid cartilage, and the level VB nodes are located between the cricoid cartilage and the clavicle. A key point is that the level IIB, III, and IV nodes are all located adjacent to the sternocleidomastoid muscle, whereas the level V nodes are located posterior to the sternocleidomastoid muscle. The level VI nodes, the upper visceral nodes, are located at the level of the hyoid bone, anterior to the sternocleidomastoid muscle and between the cricoid cartilage and the top of the manubrium. The level VII nodes, the superior mediastinal nodes, lie below the top of the manubrium and between the left and right common carotid arteries (13). Some facial nodes are not classified within the 7 levels but are described by their anatomic locations, for example, the preauricular, postauricular, and parotid nodes.
Level system of cervical lymph node classification. (Reprinted with permission of (11).)
TECHNICAL PROCEDURE FOR PLANAR LYMPHOSCINTIGRAPHY
Preoperative lymphoscintigraphy begins with an intradermal injection near the known melanoma lesion of 18.5 MBq (0.5 mCi) of filtered (0.22-μm filter) 99mTc-sulfur colloid in a 0.2-mL volume. Dynamic images of the anterior and posterior head and neck are acquired at 30 s per frame for 15 min (total of 30 frames) to ensure that the sentinel lymph node is identified. After 15 min of dynamic imaging, 5-min planar anterior and posterior images of the head and neck and of the neck and chest are acquired in addition to 3-min transmission images with a low-activity 57Co sheet source. True lateral images of the head and neck are obtained with and without transmission in addition to a true lateral marker image. It is important to acquire a lateral image of lymph node uptake. If bilateral lymphatic drainage is visualized, then lateral marker images of each side should be obtained.
Dynamic imaging is essential to permit differentiation of the sentinel lymph node from other nodes because the injected tracer travels very fast in the head and neck region. Planar imaging of the anterior and posterior head and neck, neck and chest, and lateral head and neck is essential to ensure the visualization of lymphatic drainage basins. The lateral head and neck marker image is of utmost importance for lymph node classification, especially when a hybrid SPECT/CT system is not available at the facility.
One method of acquiring a lateral marker image is to use a 5.55-MBq (150-μCi) 57Co flexible marker and two 1.85-MBq (50-μCi) 57Co disk markers. With this method, it is important to acquire a true lateral image (the patient should lie on his or her side to ensure that the head does not tilt; alternatively, the patient may stay supine and the camera can be rotated to 90° if a head immobilization method is used) with the flexible marker taped at the facial inflection points (between the nose and the forehead, between the nose and the upper lip, at the tip of the chin, at the mandibular angle, behind the ear [mastoid], and behind the neck [occipital]). One 57Co disk marker should be taped at the thyroid cartilage, and the other should be taped at the suprasternal notch. The markers must be placed correctly to ensure accurate and useful anatomic referencing (Fig. 3). Precise lymph node classification by planar imaging can become complicated if a lateral marker image is rendered useless by masking of the radiotracer at the injection site (Fig. 4) or if a true lateral image is not obtained because of slight head-angle variations in an uncomfortable or uncooperative patient. Hybrid SPECT/CT lymphoscintigraphic imaging is recommended if the location of a lymph node is unclear. If contamination is suspected because a hot site appears in a nonnodal region or unusual location, then the technologist should wipe the suspected area with a wash cloth. Planar images should be obtained after decontamination of the suspected area. If the hot uptake still appears, then SPECT/CT should be used to obtain axial images that will demonstrate activity at the surface or in a nodal area.
(A and B) Proper placement of 57Co markers before lateral marker image is obtained. Flexible marker should be taped at facial inflection points. Disk markers should be placed at thyroid cartilage and suprasternal notch. (C) Reference marker placement. Proper placement of flexible marker taped at facial inflection points to obtain reference points. Reference points are suprasternal notch (1), thyroid cartilage (2), chin (3), mandibular angle (4), mastoid (5), and occipital area (6).
Two lymph nodes are visualized on anterior and posterior head and neck images obtained with and without transmission, but on left lateral image, only 1 lymph node is visualized because of masking from radiotracer at injection site. Left lateral image for anatomic referencing is therefore not useful in this case.
HYBRID SPECT/CT IMAGING
The fusion of nuclear medicine and CT imaging is rapidly becoming a tool for precise functional and anatomic localization. In patients with cutaneous melanomas of the head and neck, hybrid SPECT/CT lymphoscintigraphic imaging is used to precisely identify the locations of sentinel lymph nodes by making use of anatomic landmarks to enhance communication between the clinician and the radiologist (6). Hybrid SPECT/CT lymphoscintigraphic imaging is performed with a system composed of a dual-head γ-camera and a CT scanner. Two types of SPECT/CT hybrid scanners are available. A hybrid system consisting of a dual-head γ-camera and a low-dose x-ray tube installed in the gantry yields low-resolution anatomic images, and a dual-head γ-camera combined with a diagnostic CT system generates high-quality anatomic images to be fused with functional images. With either system, the patient should lie supine with the arms down. The head should be immobilized to minimize head movement during the acquisition to ensure optimal fused images. SPECT images are acquired with a matrix size of 128 × 128 and 22 s per view over 180°. CT parameters may vary depending on the type of system used and the site protocol.
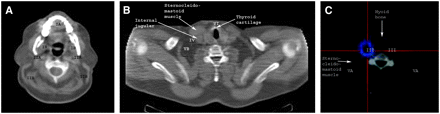
Familiarity with the most important anatomic landmarks on axial CT images is desirable for proper lymph node level classification. Figure 5 shows the most important structures. Figure 6 shows the areas where certain lymph node levels would be positioned with reference to the anatomic structures. Important structures subdividing the lymph node levels on SPECT/CT images are the mandibular angle, submandibular gland, hyoid bone, cricoid cartilage, sternocleidomastoid muscle, and internal jugular vein. The cases illustrated in Figures 7–10⇓⇓ demonstrate the value of performing SPECT/CT in addition to planar imaging for precise lymph node level classification.
Axial CT anatomy of most important structures needed for lymph node level classification.
Referencing of imaging-based lymph node level classification on axial CT imaging (A and B) and fused SPECT/CT (C).
Preauricular melanoma. (A and B) On SPECT/CT, level IIA lymph node (A) and preauricular node (B) are precisely localized. (C and D) Localization is approximate on planar image (C) because optimal linear marker image (D) references suprasternal notch (1), thyroid cartilage (2), chin (3), mandibular angle (4), mastoid (5), and occipital area (6).
Right cheek melanoma. SPECT/CT can accurately differentiate sublevels by showing CT landmarks of mandible, hyoid bone, and sternocleidomastoid muscle (B and D). Level IB lymph node (A) is located above hyoid bone and below mandible, whereas level IIA node (C) is located at mandibular angle above hyoid bone and anterior to sternocleidomastoid muscle. On planar imaging (F), jugular levels are not well defined, whereas SPECT/CT (E) encompasses structures needed to classify level III node.
Left neck melanoma. Contamination is very common during subcutaneous radionuclide injection. Therefore, when an unusual contralateral lymph node is visualized on planar imaging (C), the possibility of contamination should be considered. SPECT/CT is helpful in ruling out contamination. In this case, SPECT/CT precisely demonstrated that unusual contralateral node was level III node (B) residing between hyoid bone and sternocleidomastoid muscle (A).
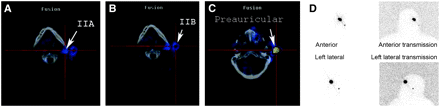
Sebaceous cell carcinoma of left upper eyelid: Planar imaging (D) demonstrated only 1 node, whereas SPECT/CT demonstrated 4 nodes, possibly because of slight delay in imaging time. Level IIA (A), level IIB (B), and preauricular (C) lymphatic chains are shown.
CONCLUSION
With conventional dynamic and planar imaging for head and neck lymphoscintigraphy, it can be very difficult to precisely classify each lymph node without anatomic references. Lateral marker images can aid in lymph node localization, but marker images are not the most reliable methods of anatomic referencing. Poor patient cooperation, problems with patient positioning or marker placement, and ambiguous lymphatic drainage patterns can all render marker images unhelpful. Hybrid SPECT/CT is the ideal tool for accurately revealing the relationship between sentinel lymph nodes and anatomic landmarks and for helping to determine at which lymph node level or sublevel a sentinel node is located. SPECT/CT facilitates the localization of sentinel nodes that are close to the radiotracer injection site and helps to rule out questionable contamination, which is common during injection.
Footnotes
-
↵* NOTE: FOR CE CREDIT, YOU CAN ACCESS THIS ACTIVITY THROUGH THE SNM WEB SITE (http://www.snm.org/ce_online) THROUGH MARCH 2008.
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication February 10, 2006.
- Accepted for publication October 4, 2006.