Abstract
We present the case report of a 72-y-old woman who underwent 99mTc-sestamibi gated myocardial perfusion SPECT with a 2-d protocol. SPECT images revealed ischemia of the apical, anteroapical, apicoseptal, and septal walls. Postdipyridamole gated SPECT revealed significant deterioration in the left ventricular ejection fraction (LVEF), wall motion, and systolic wall thickening relative to the findings obtained with rest gated SPECT. Myocardial stunning is a lingering contractile dysfunction that occurs after a brief ischemic insult. Myocardial stunning after dynamic exercise or pharmacologic stress tests has been demonstrated. Thus, the use of gated SPECT in both phases of perfusion studies may add useful information about cardiac function, as a poststress study alone probably reflects stunned myocardium in some patients undergoing ischemic stress tests. The difference between poststress LVEF and rest LVEF may have a powerful impact on prognosis, as it seems to depend on the extent and severity of induced ischemia.
The term “stunned myocardium” has been used to describe myocardium with persistent contractile dysfunction despite the restoration of perfusion after a period of ischemia; this condition usually improves with time (1).
Electrocardiography (ECG)-gated SPECT myocardial perfusion imaging can be used for the simultaneous assessment of myocardial perfusion and left ventricular function (2,3). This integrated approach has already proved useful clinically for tissue characterization and prediction of prognosis (4).
CASE REPORT
A 72-y-old woman with a history of atypical chest pain and exertional dyspnea was referred for gated myocardial perfusion SPECT. Cardiac risk factors included hypertension, diabetes mellitus for 10 y, hypercholesterolemia, and age. The results of resting ECG were normal. The patient underwent myocardial perfusion SPECT with a 2-d protocol. On the first day, 740 MBq of 99mTc-sestamibi were injected intravenously 4 min after the infusion of dipyridamole at 0.568 mg/kg. At 90 min after the injection of 99mTc-sestamibi, gated perfusion imaging was performed in the supine position by use of a dual-head γ-camera in the 90° setting (Dual-Head Variable-Angle E.CAM; Siemens) and equipped with high-resolution, low-energy collimators. After dipyridamole injection, the patient experienced no chest pain; ECG showed ST depression in the precordial leads. On the next day, at 10 min after the oral administration of a trinitroglycerin (NTG) pearl, 740 MBq of 99mTc-sestamibi were injected, and rest images were acquired with the same protocol.
The images were stored in a 64 × 64 matrix in the computer and reconstructed by filtered backprojection with a Butterworth filter (order = 5, cutoff frequency = 0.4). Gated SPECT data were analyzed with Quantitative Gated SPECT software (Cedars Cardiac Quantification; Cedars-Sinai Medical Center) for end-diastolic volume (EDV), end-systolic volume (ESV), and left ventricular ejection fraction (LVEF). Myocardial perfusion was assessed visually and semiquantitatively. A 17-segment 5-point scoring system was used for the semiquantitative assessment of myocardial perfusion.
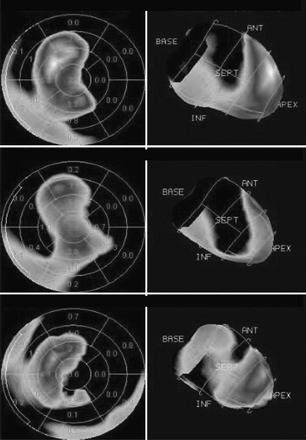
Reversible defects (myocardial ischemia) were noted in the apical, anteroapical, apicoseptal, and anteroseptal walls (Figs. 1 and 2). The summed stress score, summed rest score, and summed difference score were 11, 5, and 6, respectively.
Abnormal 99mTc-sestamibi myocardial perfusion SPECT. Images show large reversible defect involving apical, anteroapical, apicoseptal, septal, and anteroseptal walls.
Three perfusion polar maps (from top to bottom: stress, rest, and reversibility) show extent and severity of reversible perfusion defect. ANT = anterior; INF = inferior; SEPT = septal.
The rest gated images displayed in 2-dimensional and 3-dimensional (Fig. 3) formats revealed normal left ventricular wall motion and systolic wall thickening. EDV, ESV, and LVEF calculated from the rest gated images were 53 mL, 17 mL, and 68%, respectively. The stress gated images acquired after dipyridamole injection (Fig. 3) revealed akinesia in the apicoseptal region as well as hypokinesia in the apical, anteroapical, anteroseptal, and septal walls. Calculated EDV, ESV, and LVEF were 69 mL, 46 mL, and 33%, respectively.
Three-dimensional display of ventricular wall motion in postdipyridamole and rest gated images. Poststress images reveal remarkable wall motion abnormality in apex and septum, whereas rest images reveal normal left ventricular wall motion. Wire-frame and shaded surfaces represent endocardium in end-diastolic and end-systolic phases, respectively. ANT = anterior; INF = inferior; LAT = lateral; SEPT = septal.
Dipyridamole gated 99mTc-sestamibi SPECT revealed significant deterioration in systolic myocardial function (decreased LVEF) as well as remarkable wall motion and systolic wall thickening abnormalities (myocardial stunning) in the regions of reversible defects relative to the findings obtained with rest gated SPECT.
DISCUSSION
Myocardial stunning is a lingering contractile dysfunction that occurs after a brief ischemic insult, even in the absence of necrosis, and persists for some time after the restoration of adequate blood flow (1). The histologic appearance of the stunned myocardium is normal, so there is no permanent damage and contractile function is able to recover gradually with time (5). Although in some instances full recovery may occur within a few minutes after the recovery of myocardial perfusion, in some cases it may take hours, days, or even weeks, depending on the severity of the ischemic episode (6). A variety of hypotheses have been postulated as mechanisms for myocardial stunning; these include insufficient energy production by mitochondria, impaired energy use by myofibrils, reduced myofilament responsiveness to calcium, reduced contractile protein activation, calcium overload, and generation of free radicals (6,7). Reperfusion through the restoration of the oxygen supply, which is beneficial and protective for the ischemic myocardium, induces at the same time a burst of oxygen-free radicals, complicated by intracellular Ca2+ overload, which is harmful for contractile proteins (7).
Well-documented clinical settings in which myocardial stunning can occur include percutaneous transluminal coronary angioplasty, unstable and variant angina, acute myocardial infarction with early reperfusion, cardiac surgery, and cardiac transplantation (8,9). However, myocardial stunning also can develop after silent or symptomatic ischemic episodes during common daily activities and after diagnostic stress tests with an ischemic response (1,10–12).
Barnes et al. (10) demonstrated impaired ventricular function 45 min after dobutamine-induced ischemia, which worsened after a second ischemic episode, confirming that repeated episodes of ischemia can lead to cumulative myocardial stunning. In another study, Barnes et al. (13) demonstrated that stunning is related to the severity of coronary artery stenosis. Using MRI, Kraitchman et al. (14) showed persistent myocardial dysfunction up to 30 min after reperfusion in a closed-chest canine model of myocardial stunning; they also reported a graded response in systolic function related to the severity of the induced ischemia.
Sestamibi labeled with 99mTc is a suitable agent both for detecting perfusion defects and for delineating endocardium edges for the assessment of ventricular function in the gated SPECT mode. Therefore, 99mTc-sestamibi provides information about both hypoperfusion and stunning through separate mechanisms: by means of tracer distribution in the myocardium demonstrating flow heterogeneities and by means of systolic ventricular parameters showing transient dysfunction. Thus, both the cause and the consequence are simultaneously evaluated in a single study (6).
Although dynamic exercise and dobutamine tests are considered to be the procedures most capable of provoking myocardial ischemia, dipyridamole-induced myocardial stunning recently was demonstrated (1,6,15). Adenosine-induced stunning also has been reported (12), confirming that vasodilators are capable of producing more than simple flow heterogeneity.
LVEF as measured by gated SPECT slightly but significantly decreases in the poststress period when an ischemic insult is present, whereas it remains mostly unchanged, with a slight tendency to increase, when ischemia is absent (3,6). A reduction in LVEF after stress seems to be related to an increase in ESV. The inadequate contraction may cause the increase in ESV (2,16). It has been demonstrated that patients with myocardial stunning have more severe clinical, angiographic, perfusion, and function findings (2,16), suggesting that the identification of this phenomenon may have a potential impact on risk stratification and prognosis. The best predictor of stunning after stress is stress-induced ischemia, and its occurrence is related to the degree of ischemia (1,3,11,16).
False-negative results in myocardial perfusion imaging may be encountered if all of the myocardial territories are uniformly affected. Ischemic stunning after dipyridamole stress in gated SPECT may be an indicator of severe and extensive coronary artery disease and can aid in the interpretation of borderline perfusion images and the elimination of false-negative results secondary to relatively balanced lesions in 3-vessel disease (17).
We routinely administer a pearl of NTG at rest 10 min before radiotracer injection in all patients who have any perfusion abnormality on stress images. Pepine et al. (18) studied the effect of intravenous NTG infusion on LVEF in a group of asymptomatic patients who had coronary artery disease and left ventricular wall motion abnormalities. The authors showed a mean increase of 3.7% in LVEF after intravenous NTG administration in all patients and a mean increase of 9.4% in patients with wall motion improvement (18). Other researchers observed a tendency for an increase in the segmental ejection fraction after NTG infusion in patients with ischemic heart disease (19). However, the administration of sublingual NTG (0.8 mg) did not change global LVEF at rest in a study of patients with isolated disease of the left anterior descending artery (20). Because of the short half-life of the NTG pearl and because we performed rest imaging 100 min after oral NTG administration, it is less likely that such a large change in the ejection fraction could be explained by an effect of NTG.
We conclude that the use of gated SPECT in both phases of perfusion studies may add useful information about cardiac function, as a poststress study alone probably reflects stunned myocardium in patients undergoing ischemic stress tests. In this setting, the difference between poststress LVEF and rest LVEF represents a new quantitative parameter derived from gated SPECT studies to be included in the investigation of patients with coronary artery disease (6). In addition, it may be demonstrated to have a powerful impact on prognosis, as it seems to depend on the extent and severity of induced ischemia (1,3,11,16).
References
- Received for publication November 9, 2005.
- Accepted for publication March 20, 2006.