Abstract
Objective:Measurement of cardiac perfusion via agents such as 99mTc-sestamibi (Cardiolite; DuPont-Merck Pharmaceutical Co., Inc.) is widely used in clinical nuclear medicine for the diagnosis of coronary artery disease. The monograph for 99mTc-sestamibi recommends at least 90% radiochemical purity (RCP) for clinical use. Various factors may influence the RCP of certain reagent kits. Some of these include the amount of activity added to the reagent kit, the generator ingrowth time, the generator manufacturer, the age of the eluate, and the age of the formulated kit. A D-optimal design with a 20-experiment run was devised to study the effects of these variables either alone or in combination on the RCP of 99mTc-sestamibi.
Methods:The RCP was assessed by Baker-Flex thin-layer and high-performance liquid chromatographic methods, immediately and 6 h after reconstitution of the 99mTc-sestamibi.
Results:The results showed that 4 of the 5 variables studied were statistically significant predictors of the RCP. The age of the formulated kit did not influence the RCP.
Conclusion:For any combination of these 4 variables, the mean RCP remained greater than or equal to 90%, that is, within the recommended range of RCP for clinical use at radioactivity levels ranging from 5,550 MBq to 37,000 MBq.
Noninvasive nuclear medicine imaging can be used effectively in the diagnosis of patients with coronary artery disease (1,2). Although cardiac imaging is broad in its scope, a primary focus has been on the assessment of myocardial perfusion (2), which is of clinical significance, since the precise measurement of regional myocardial perfusion in humans can identify ischemia. In addition, these methods define the extent and severity of disease, assess the myocardial viability, and establish the need for medical/surgical intervention (2).
Most clinical nuclear medicine cardiac studies use SPECT for image acquisition (3), although some larger centers have access to PET (4). Since its introduction in 1975, 201Tl-thallous chloride has been used as the main radiopharmaceutical for evaluation of myocardial perfusion (5–7). Because of several drawbacks in the biologic and physical properties of 201Tl, cardiac perfusion imaging agents labeled with 99mTc have been developed (8,9). These agents include 99mTc-sestamibi (Cardiolite; DuPont-Merck Pharmaceutical Co., Inc.) and 99mTc-tetrofosmin (Myoview; Amersham Health).
99mTc-Sestamibi is a monovalent, cationic, lipophilic complex that consists of 1 atom of 99mTc in a +1 oxidation state and 6 molecules of 2-methoxyisobutylisonitrile (MIBI) (10). According to the product package insert, 99mTc-sestamibi is prepared by the addition of up to 5,550 MBq of 99mTc-sodium pertechnetate to a lyophilized kit containing the MIBI ligand, in the form of tetrakis (2-MIBI) copper (I) tetrafluoroborate (11,12). Stannous chloride dihydrate and tin chloride (stannous and stannic) are used as the reducing agents. The kit contains other adjuvants such as l-cysteine hydrochloride monohydrate, sodium citrate dihydrate, and mannitol (11,12). This lyophilized, sterile, nonpyrogenic preparation is stored under nitrogen headspace. Before lyophilization, the pH of the preparation is adjusted to between 5.3 and 5.9. After reconstitution, the pH is between 5.0 and 6.0. The lyophilized kit is stable at room temperature and can be stored for up to 18 mo from the date of manufacture (11).
The package insert for 99mTc-sestamibi gives specific recommendations for the quality control of the radiolabeled product (12). Several factors may potentially affect the radiochemical purity (RCP) of the 99mTc-sestamibi. Studies performed by other investigators and the preliminary work performed in our laboratories has pointed out that such factors include the amount of activity added to the reagent kits and the age of the reconstituted product (13–21). The Baker-Flex (Baker, Inc.) thin-layer chromatography (TLC) method and several other techniques can be used to verify the RCP of 99mTc-sestamibi. The preliminary work that has been performed in our laboratories included the assessment of the RCP of 99mTc-sestamibi reconstituted at various levels of activity and for up to 12 h after preparation, by several methods.
The quality control methods included solvent saturation pad chromatography (13), high-performance liquid chromatography (HPLC) (13), and TLC (11,12). Although these preliminary studies provided interesting data on the effects of the activity added to the reagent kit and the age of the radiolabeled product on the RCP, some important variables such as the generator manufacturer, generator ingrowth time, and eluate age were not included. Also, the combined effects of generator manufacturer, generator ingrowth time, the activity added to the reagent kit, the age of the radiolabeled product, and the eluate age on the RCP were not studied. It is important to understand these factors and their combined influence on the RCP of 99mTc-sestamibi, since this will allow clinicians to predict product quality with greater accuracy. Because our preliminary work provided only limited information, the present study was designed to evaluate a wide variety of factors that can influence the RCP of 99mTc-sestamibi.
MATERIALS AND METHODS
The lot numbers and vendors for the chemicals and equipment used are given below.
Baker-Flex aluminum oxide plates, 2.5 × 7.5 cm, 1B-F, lot H; J.T. Baker, Inc.
Trifluoro acetic acid, lot 933262; Fisher Scientific
Acetonitrile Omnisolv, HPLC grade, lot 33012; EM Sciences
Chloroform, HPLC grade, lot 931412; Fisher Scientific
Cardiolite reagent kits, lot SJ 12441/A; DuPont-Merck Pharmaceutical Co., Inc.
Absolute Alcohol, lot 6810-00-242-3645; Midwest Grain Products, Inc.
Sep-Pak cartridges, lot P3035A1; Waters
Nylon membrane filters, 47 mm, 0.45 μm, lot 3012502; Gelman Science
C-18 Microbondapak, 12.5 nm, 10-μm column, 3.9 × 300 mm, part 27324, serial P31941C15; Waters
99Mo generators:
DuPont-Merck lot G-9344-6-E 6, 7, 8 and G-9345-6-E 6, 7, 8; DuPont-Merck Pharmaceutical Co., Inc.
Mallinckrodt lot 1073046M 1, 2 and 1073047M 1, 2; Mallinckrodt Medical
Medi-Physics lot 3507333401 and lot 3507333471; Medi-Physics
Integrator recorder 3390-A; Hewlett-Packard Co.
420 controller; Altech
160 ultraviolet absorbance detector; Beckman
110 A HPLC pumps; Beckman
AP-2H scaler-timer; Berkeley Nucleonics Corp.
Radioisotope calibrator CRC-12, Capintec
Series 35 Plus multichannel analyzer and strip scanner; Canberra
RS/1 software; BBN Technologies
Experimental Design
Response surface modeling from a software package, RS/1, was used to study the effect of the stated variables on RCP levels. The following 4 factors were evaluated: generator ingrowth time (24, 48, and 72 h), activity added to the reagent kit (5,550, 21,275, and 37,000 MBq), generator manufacturer (DuPont-Merck, Medi-Physics, and Mallinckrodt Medical), and eluate age (0, 3, and 6 h). Our study was limited to these 4 selected variables and did not include other variables known to affect radiochemical impurities. These additional factors include different heating methods, heating temperature, and heating time and have been discussed extensively in several published articles (13,20–26). For evaluation, these 4 factors at 3 levels would normally require 81 (3 × 3 × 3 × 3) separate experiments using a standard experimental design. In order to be cost efficient and yet accurately estimate the effects of the 4 factors under study, we used a D-optimal design technique to arrive at a smaller number of required runs rather than a standard classic design. Experimental designs using D-optimal algorithms are one form of computer-aided methodology that is particularly useful when classic designs do not apply (27,28). Our experimental design was based on a hypothetical dataset generated from the equation that included the main effects of each factor and an interaction term between generator ingrowth time and generator manufacturer. The equation and the design were developed and tested by scientists at DuPont-Merck Pharmaceutical Co., Inc.:
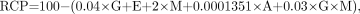 Eq. 1 where G = generator ingrowth time (in hours), E = eluate age (in hours), A = activity (in megabecquerels), and M = generator manufacturer (Mallinckrodt, Medi-Physics, or DuPont-Merck).
Eq. 1 where G = generator ingrowth time (in hours), E = eluate age (in hours), A = activity (in megabecquerels), and M = generator manufacturer (Mallinckrodt, Medi-Physics, or DuPont-Merck).
Using the above equation, “theoretic” RCP values were generated and 2% random noise was added to introduce variability. This simulation study was done in order to arrive at a D-optimal design requiring fewer runs but accurately identifying the factors being studied. Analysis of the simulated data from a 20-run D-optimal design provided an excellent fit to the hypothetical equation. The D-optimal design with 20 runs as specified in Table 1 was therefore adopted for this study.
Twenty-Experiment Run Design
Both the Baker-Flex TLC method and the HPLC method were used to evaluate the RCP of the labeled 99mTc-sestamibi. The RCP values obtained using both these methods were then used for the analysis of data to show the effect of these 4 variables and their interactions on the RCP.
In addition to the 4 factors mentioned in the hypothesis, it was of interest to evaluate the effect of the age of the reconstituted 99mTc-sestamibi on the RCP. Therefore, the 20-run experimental design was conducted at times 0 and 6 h after reconstitution. However, this factor was not included in the design of the hypothetical equation and in generating the 20-run experiment. We decided to evaluate this factor as an additional variable in our study based on earlier data and the package insert information, which indicates the shelf life of the reconstituted product to be no more than 6 h (12). Effectively, this created a fifth variable in the final model at the data analysis stage. For each of the 20 experiment time points, 2 99mTc-sestamibi kits were formulated. The data sets obtained from the 2 kits that were prepared and tested are shown in Tables 2–4 as kit preparation 1 and kit preparation 2. The RCP of these kits was assessed at 0 and 6 h using the Baker Flex TLC method (in triplicate) and the HPLC method (single sample).
Baker-Flex TLC RCP at 0 Hours
Baker-Flex TLC RCP at 6 Hours
HPLC RCP at 0 and 6 Hours
Baker-Flex TLC Method
RCP was determined using methods and materials recommended in the package insert (12).
HPLC Analysis
Gradient HPLC continuously monitored the effluent by a radiometric detector with the output automatically integrated. The stationary phase was a 3.9 (internal diameter) × 300 mm Microbondapak 12.5 nm, 10 μm C-18 column (Waters). The mobile phase had a flow rate of 1.5 mL/min. Solvent A was 700:300:1 water:acetonitrile:trifluoroacetic acid (TFA); solvent B was 100:900:1 water:acetonitrile:TFA. The mobile phase consisted of a gradient system: 100% solvent A to 100% solvent B over 10 min, 100% solvent B for 1 min, a return to 100% solvent A over 1 min, and equilibration at 100% solvent A for 6 additional minutes. The total run time was 18 min.
Using this method, all intermediates and by-products were eluted before the 99mTc-sestamibi peak at 9–11 min, with the exception of 99mTc-dimethyl vinyl isonitrile, which was usually <1% and came off the column just after the 99mTc-sestamibi peak. The HPLC was validated by both interday and intraday standards to achieve data robustness. 99mTc-Sestamibi kits with both high and low RCP were prepared to determine that the quality control of the instruments and methods used in this design was satisfactory.
RESULTS
Tables 2–4 summarize the observed RCP values and their means using both the Baker-Flex TLC and the HPLC methods. The RCP values are given for each of the 2 methods for the 2 times at which samples were tested, that is, 0 and 6 h after reconstitution of the reagent kits. Separate models were run for the 2 times. The final model included a factor for the age of the reconstituted 99mTc-sestamibi to test whether age affected the RCP. The factor for age was included both as a main factor and as an interaction term. Table 5 shows the predicted RCP values for all 81 possible combinations, whereas Table 6 shows the predicted values for the RCP based on the final model, which includes all of the original 4 factors—generator ingrowth time, generator manufacturer, activity added to the reagent kits, and eluate age—and the added fifth factor—age of the reconstituted product for the 20-experiment run. It was observed from the data in Tables 2–4 that the age of the reconstituted product was not a significant factor for the prediction of RCP values. We were therefore able to pool the replicate sets of data for 0 and 6 h into a single set of data in which the mean RCP obtained from the Baker-Flex TLC and the HPLC methods was used. ANOVA for a D-optimal design was the statistical method used to analyze the data. The final model equation is given below. Each of the individual terms is the same as that listed in the model equation described earlier. The terms with subscripts (A1, B1–B4, and C1–C6) are the coefficients generated from the RS/1 software.
Predicted RCP Values for All 81 Experiments Using Equation with Coefficients for Baker-Flex TLC and HPLC Methods
Observed and Predicted Values of RCP for 20-Experiment Run
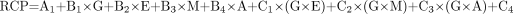 Eq. 2
Eq. 2

The actual equations for each of the 2 methods for the RCP evaluation with the appropriate coefficients generated from the RS/1 software for the generator manufacturers are shown below.
HPLC Method
For the Mallinckrodt generator, the equation is as follows:
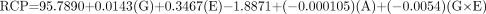
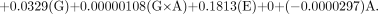
For the Medi-Physics generator, the equation is as follows:
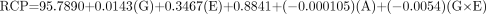
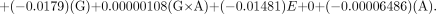
For the DuPont-Merck generator, the equation is as follows:

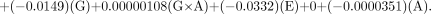
Baker-Flex Method
For the Mallinckrodt generator, the equation is as follows:

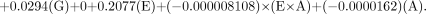
For the Medi-Physics generator, the equation is as follows:
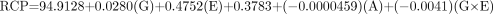
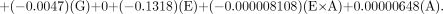
For the DuPont-Merck generator, the equation is as follows:
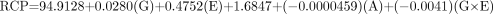
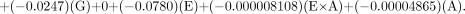
Table 6 shows the observed and predicted RCP values for the different combinations of variables examined. The 4 factors initially identified made a statistically significant contribution to the predicted RCP values. The fit of the model was measured by the R and R2 adjusted terms. R2 adjusted is the R2 value that has been adjusted to reflect the “true” fit of the data to the model, accounting for the fact that every term in the model decreases the lack of fit whether or not it is significant. For the 2 methods of testing RCP, that is, the Baker-Flex TLC and the HPLC methods, the R2 values were 0.79 and 0.74, respectively. The R2 adjusted values were 0.69 and 0.74, respectively. To test the goodness of fit of the model, the lack-of-fit term was evaluated with ANOVA. The ANOVA evaluation of the data for the final model was found to be statistically insignificant both for the HPLC method (P = 0.93) and for the Baker-Flex TLC method (P = 0.99), indicating that the model fit the data adequately.
DISCUSSION
From our analysis, the factors that are important in predicting RCP are generator ingrowth time (24–72 h), generator manufacturer, eluate age (0–6 h), and activity added to the reagent kit (up to 37,000 MBq). These factors, either alone or in combination, have a statistically significant effect on the RCP of the formulation after the radiolabeling of 99mTc-sestamibi. This conclusion is based on the results of the experiments given in Table 6 and the predicted RCP values calculated for all 81 possible combinations in Table 5. The time after reconstitution of the formulation was not a significant predictor of the RCP values of the formulation either alone or in combination with other factors. However, from a clinical standpoint, it is important to note that these factors, alone or in combination, do not cause RCP to fall below the recommended level of 90%. Under all possible combinations of the variables investigated, the predicted RCP values were always greater than 90% (which is the requirement for use in a clinical setting) with one noted exception. A single HPLC analysis showed a labeling efficiency of only 89.95% although the mean HPLC RCP and the TLC RCP for these samples were greater than 90%. Using the equations developed for the 3 generator manufacturers, RCP can be predicted to a reasonable degree of accuracy. However, the actual RCP values may vary depending on the analytic method used.
Several investigators have studied the effects of different variables on RCP. One group of researchers evaluated different techniques for heating the kits (15). We used only the heating method described in the package insert (12) and therefore cannot directly compare our results to these findings. Several other studies evaluated the effects of fractionating the kit before radiolabeling and the resultant RCP on radiolabeling of the fractionated kits (16–18). We evaluated only the intact kit and not the effects of splitting the kit or freezing and thawing aliquots of the cold kit. We also did not use the first elution of the generator in our study and therefore cannot directly compare our findings with those of researchers who used the first elution from long generator ingrowth times at different activities at 3–12 h after elution (19–21).
CONCLUSION
The 99mTc-sestamibi kits in our study were reconstituted with various levels of radioactivity. After radiolabeling, samples were taken from the kit vials immediately and at 6 h and then were tested for RCP. Triplicate samples were analyzed at each time point for TLC, whereas only a single sample was run for each time point on HPLC. Each method gave similar results for RCP. RCP tests indicated a greater than 90% labeling efficiency for 99mTc-sestamibi up to 6 h after radiolabeling at activity levels ranging from 5,550 to 37,000 MBq.
Acknowledgments
This research was partially supported through an unrestricted research grant from DuPont-Merck Pharmaceutical Co., Inc. (now Bristol-Myers Squibb Medical Imaging).
Footnotes
For correspondence or reprints contact: Jeffrey P. Norenberg, PharmD, College of Pharmacy, MSC09 5360, 1 University of New Mexico, Albuquerque, NM 87131-0001.
E-mail: jpnoren{at}unm.edu







