Abstract
Our objective was to assess the renal toxicity profile of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with a metastatic neuroendocrine tumor (NET) and a single functioning kidney. Methods: This was a retrospective analysis of NET patients who had undergone 177Lu-DOTATATE PRRT at a large tertiary-care center. All patients selected for the study had somatostatin receptor–positive NETs, had received at least 3 cycles of 177Lu-DOTATATE PRRT, and had a documented single functioning kidney. The analyzed parameters included patient characteristics, metastatic burden, renal characteristics at diagnosis and during therapy, and nephrotoxic factors. For the renal assessment, the following characteristics were studied before each PRRT cycle: glomerular filtration rate (GFR) as estimated by 99mTc-diethylenetriamine pentaacetic acid renography, effective renal plasma flow (ERPF) as measured by 99mTc-ethylenedicysteine renography, and blood urea and serum creatinine levels. Renal toxicity was evaluated using version 4.0 of the Common Terminology Criteria for Adverse Events (NCI-CTCAE score). The percentage reduction in GFR and ERPF was also assessed. Filtration fraction was calculated to clarify whether there was a relatively greater reduction in one index of renal function than in the other. Results: At the time of analysis, 6 patients met the inclusion criteria, having received between 3 and 5 cycles of therapy with a cumulative activity of 16.6–36.2 GBq. The duration of follow-up ranged from 12 to 56 mo. The overall toxicity profile (as per the NCI-CTCAE score) showed no acute renal toxicity in any patient. Regarding overall chronic renal toxicity, 3 patients had none, 1 patient had grade II, and 2 patients had grade I. All patients with overall chronic renal toxicity showed compromised renal function at the outset (baseline). The 2 patients with grade I chronic renal toxicity after PRRT had grade II at baseline and gradual improvement over the subsequent cycles. One patient with grade II at baseline showed transient worsening to grade III after the first cycle followed by gradual improvement and a return to baseline after the second cycle. Only 2 patients showed a reduction in GFR (5.3% in one and 13.84% in the other). Four patients showed a reduction in ERPF (31.4% in the patient with the greatest reduction), and all had a rise in filtration fraction signifying that tubular parameters were more affected than glomerular parameters. Conclusion: With proper renal protection and dose fractionation, it is feasible to use 177Lu-DOTATATE PRRT in patients with NET and a single functioning kidney. Further studies are required to assess the long-term renal consequences of changes in ERPF and filtration fraction in these patients.
- neuroendocrine tumor
- bone marrow metastasis
- peptide receptor radionuclide therapy (PRRT)
- 177Lu-DOTATATE
Peptide receptor radionuclide therapy (PRRT) with 177Lu- or 90Y-labeled somatostatin receptor analogs has been widely used for targeted treatment of metastatic or inoperable neuroendocrine tumors (NETs). Absorbed dose not only to bone marrow but to the kidney is a well-perceived limitation of PRRT, with dose-related renal toxicity being documented in the literature (1–3). Patients with a single functioning kidney form a distinct subset that obviously can create clinical concern about tolerability. Hence, in this subset it is imperative to assess the risk and feasibility of PRRT by observing and accruing the renal profile data after treatment.
The premise of this retrospective analysis was to evaluate the renal profile (focusing primarily on renal toxicity) of this particular subset of patients selected from those who had undergone PRRT over the last 5 y in a large tertiary care center. For an adequate assessment of the toxicity profile, patients who had received at least 3 cycles of PRRT were selected. In our analysis, however, there was no patient of this particular subgroup who received less than 3 cycles or for whom PRRT was terminated with fewer cycles at the time of the study.
MATERIALS AND METHODS
The study cohort was selected from 295 patients treated over the last 5 y. All had somatostatin receptor–positive NETs, a single functioning kidney, and at least 3 cycles of 177Lu-DOTATATE PRRT (5.5 GBq, because all patients had metastatic disease). All the patients underwent the single-day amino acid renal-protection protocol (designed to infuse the recommended 25 g of lysine and 25 g of arginine over 7.5–8 h, with the amino acid infusion starting 60 min before therapy). No adjustment was applied for the single functioning kidney.
The analyzed parameters included patient characteristics, metastatic burden, renal characteristics at diagnosis and during therapy, and nephrotoxic factors. Before each cycle of PRRT, glomerular filtration rate (GFR) was estimated by γ-camera–based 99mTc-diethylenetriamine pentaacetic acid renography, and effective renal plasma flow (ERPF) by 99mTc-ethylenedicysteine renography. Additionally, filtration fraction was calculated by dividing GFR by ERPF. Presentation of GFR and ERPF data in terms of filtration fraction is useful when both indices of renal function decrease (as frequently occurs in renal insufficiency) and can clarify whether there has been a greater reduction in one index than in the other (e.g., a greater reduction in ERPF would lead to an increase in filtration fraction, whereas the opposite would occur if there were a greater reduction in GFR).
Blood urea levels and serum creatinine were also evaluated at each time point, and the patient’s history regarding risk factors known to be associated with renal toxicity was assessed. During each PRRT cycle, the standard protocol was followed (3).
Renal toxicity was evaluated using version 4.0 of the Common Terminology Criteria for Adverse Events (NCI-CTCAE score) (4). According to these criteria, chronic renal toxicity is considered grade I (+) if creatinine clearance is below the lower limit of normal (60 mL/min/1.73 m2), if 2+ proteinuria is present, or if the creatinine level is greater than 0.5 mg/dL; grade II (++) if creatinine clearance is 59–30 mL/min/1.73 m2; grade III (+++) if 29–15 mL/min/1.73 m2; grade IV (++++) if below 15 mL/min/1.73 m2 (dialysis or renal transplantation indicated); and grade V (+++++) if the patient has died. Acute renal toxicity is considered grade I (+) if the creatinine level is greater than 0.3 mg/dL or 1.5–2.0 times baseline, grade II (++) if 2–3 times baseline, grade III (+++) if greater than 4.0 mg/dL or more than 3 times baseline (hospitalization indicated), grade IV (++++) if the patient’s life is threatened, and grade V (+++++) if the patient has died.
RESULTS
Of the 295 patients, 6 had a single functioning kidney (3 men and 3 women; age range, 33–63 y) (Table 1). Two of these 6 had pancreatic NET, one had renal NET, one ureteral NET, one small-bowel NET, and one metastatic NET of unknown primary. Sites of metastasis in addition to the primary were liver (4 patients), bone (2 patients), and adrenal gland (1 patient). The histologic finding was well-differentiated NET, intermediate-grade NET, and poorly differentiated NET in 2 patients each (Table 1). Five patients had a functioning right kidney and one a functioning left kidney. The single functioning kidney either was idiopathic or was caused by extension of tumor into the kidney, genitourinary tract NET (ureteral NET and horseshoe-kidney NET), or congenital anomalies of the kidney (Table 2). On somatostatin receptor imaging, 5 patients had grade IV uptake (greater than liver uptake) and one had grade III uptake (equal to liver uptake) (Table 3). The 6 patients underwent PRRT because their underlying NET caused severe systemic complaints that had not been relieved by oral medication or somatostatin analogs (Table 3).
Patient Characteristics
Cause of Single Functioning Kidney and Associated Level of Biochemical Tumor Marker
PRRT Indication and Grade of Tracer Uptake on Pretreatment Diagnostic Scan
Risk factors for nephrotoxicity were also assessed: longstanding hypertension (>10 y), diabetes mellitus (>10 y), and prior nephrotoxic chemotherapy. One patient had hypertension, one a past history of diabetes mellitus, and two a history of chemotherapy (capecitabine in one and 6 cycles of cisplatin in the other) (Table 4).
Risk Factors for Nephrotoxicity
At the time of analysis, the patients had received between 3 and 5 cycles of 177Lu-DOTATATE PRRT (cumulative activity, 16.6–36.2 GBq) (Table 4). Follow-up ranged from 12 to 56 mo. According to the NCI-CTCAE scores, no patients had acute renal toxicity but 3 had chronic renal toxicity (grade II in patient 3 and grade I in patients 4 and 6) (Table 5). These 3 patients also had compromised renal function at the outset.
Overall Renal Toxicity Profile
NCI-CTCAE scoring was repeated after each cycle (Table 6). At no time did any patient show acute renal toxicity. Two patients had transient grade I chronic renal toxicity after the first cycle of PRRT with improvement and normalization in subsequent cycles. Two patients had grade II chronic renal toxicity before PRRT that gradually improved and was reduced to grade I after PRRT. One patient had grade II chronic renal toxicity before PRRT with transient worsening to grade III after the first cycle and a gradual return to baseline after the second cycle (Table 6). One patient had no chronic renal toxicity overall or after any cycle.
Renal Toxicity Profile After Each Cycle of PRRT
The overall percentage reduction in GFR and ERPF was also assessed. Only 2 patients had a reduced GFR (by 5.3% in one and 13.9% in the other). Four patients had a reduced ERPF (31.4% in the patient with the greatest reduction) (Table 7), which was accompanied in all cases by a rise in filtration fraction, signifying that tubular parameters were more affected than glomerular parameters (Table 7; Fig. 1). The level of serum chromogranin A was evaluated as a biochemical tumor marker (Table 2).
Reduction in GFR and ERPF after PRRT and Corresponding Change in Filtration Fraction
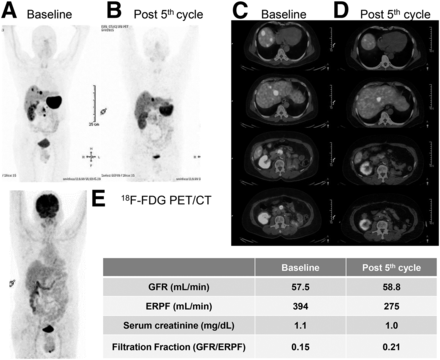
62-y-old man who initially presented with abdominal pain and had CT-documented mesenteric mass that, after excision, was found to be well-differentiated NET. Patient also had history of renal cell carcinoma of left kidney and had undergone left nephrectomy. (A and C) After disease-free period of 4 y, patient presented with disease recurrence, with 68Ga-DOTATATE PET/CT showing somatostatin receptor–expressing lesions in segments IV and VIII and a few peripancreatic nodules. (E) 18F-FDG PET/CT showed normal findings. Patient then underwent 5 cycles of 177Lu-based PRRT. (B and D) After the fifth cycle, 68Ga-DOTATATE PET/CT showed almost complete resolution of lesions in segment VIII and around the pancreas, with only the segment IV lesion seen. Overall assessment was good partial response to treatment. After 5 cycles, there was grade I chronic renal toxicity but no acute renal toxicity. Patient had same grade I chronic renal toxicity before undergoing PRRT. Baseline filtration fraction of 0.15 (rounded off) increased to 0.21 after 5 cycles of therapy because of reduction in ERFP compared with GFR (which was stable in this example). A color version of this figure is available as a supplemental file at http://tech.snmjournals.org.
DISCUSSION
PRRT is an increasingly popular therapeutic modality that is being widely used in the management of advanced NET (5). Recent guidelines state that PRRT can be used in patients with well-differentiated and intermediate-grade NETs (Ki-67 index < 20%). The guidelines of the European Society for Medical Oncology suggest that PRRT can be given for NETs with a Ki-67 index of less than 30% (6).
No overall acute renal toxicity was observed in any patient, whereas post-PRRT chronic renal toxicity was seen in 3 patients (grade II in one and grade I in two. The patient with grade II (after 5 cycles of PRRT) was at grade II even before undergoing PRRT. Two patients who showed overall grade I chronic renal toxicity had grade II chronic renal toxicity at the outset before undergoing PRRT. Three patients showed no chronic renal toxicity. In 3 patients (2 with no resultant renal toxicity and 1 with grade II renal toxicity), there was some transient deterioration of renal function after the first cycle of PRRT with improvement over subsequent cycles.
Assessment of associated nephrotoxic risk factors (longstanding hypertension, diabetes, and nephrotoxic chemotherapy) is essential in evaluating renal dysfunction after PRRT. Renal dysfunction may be aggravated in patients in whom these factors coexist (7). In our analysis, a patient who had received cisplatin chemotherapy experienced grade II chronic renal toxicity after PRRT, but this patient had compromised renal function even at baseline. Another patient who received cisplatin chemotherapy experienced transient chronic renal toxicity after the first cycle that gradually reversed to normal after the second cycle. The same patient had longstanding diabetes. A patient with longstanding hypertension experienced transient chronic renal toxicity after the first cycle that also gradually reversed to normal after the second cycle. Thus, even with a single functioning kidney and the presence of other nephrotoxic factors, there was no evidence of significant renal toxicity associated with 177Lu DOTATATE PRRT.
Positively charged amino acids such as l-lysine or l-arginine are coinfused to competitively inhibit proximal tubular reabsorption of the 177Lu-based DOTA analogs. Coadministration of these amino acids significantly reduces the absorbed renal dose, which ranges from 9% to 53% (8). Dose fractionation is also used in high-risk patients in whom renal toxicity is more likely than in healthy individuals. In all our patients, none of whom showed any acute renal toxicity, both coinfusion of amino acids and dose fractionation were applied.
More patients showed a decline in ERPF (4/6) than a decline in GFR (2/6), clearly indicating that the radiolabeled peptides affected tubular parameters more than glomerular parameters. Follow-up studies are needed to evaluate the long-term consequences to tubular toxicity of using 177Lu-DOTATATE in this group. Renal toxicity is thought to be more profound from 90Y-based PRRT than from 177Lu-based PRRT (9,10). In none of our patients did 177Lu-based PRRT show associated nephrotoxicity, indicating a safe renal profile.
CONCLUSION
This retrospective data analysis indicated that, when administered along with renal protection and dose fractionation, the use of 3–5 cycles of 177Lu-DOTATATE PRRT in NET patients with a single functioning kidney is feasible and shows no acute or chronic renal toxicity on follow-up. However, the changes in ERPF observed in these patients warrant prospective studies to assess long-term renal consequences and the renal dosimetry.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Feb. 4, 2016.
REFERENCES
- Received for publication October 13, 2015.
- Accepted for publication February 3, 2016.