Abstract
Radionuclide scintigraphy with technetium-labeled bisphosphonates has brought a paradigm shift in diagnosing cardiac amyloidosis (CA), with transthyretin CA now being effectively diagnosed without the need for tissue biopsy. Yet, deficits remain, such as methods for the noninvasive diagnosis of light-chain CA, means to detect CA early, prognostication, monitoring, and therapy response assessment. To address these issues, there has been growing interest in the development and implementation of amyloid-specific radiotracers for PET. The aim of this review is to educate the reader on these novel imaging tracers. Though still investigational, these novel tracers—given their many advantages—are clearly the future of nuclear imaging in CA.
Cardiac amyloidosis (CA) is a restrictive cardiomyopathy caused by abnormal deposition of amyloid fibrils in the extracellular space of the heart. Depending on the type of amyloid fibril deposit, 95% of CA is caused by either transthyretin amyloidosis (ATTR) or light-chain amyloidosis (AL) (1,2). Cardiac ATTR may be inherited (autosomal dominant inheritance, known as hereditary transthyretin amyloidosis) or acquired (known as wild-type transthyretin amyloidosis) (3). Cardiac AL occurs due to deposition of monoclonal light chains that are produced in excess in patients with plasma cell dyscrasias (4).
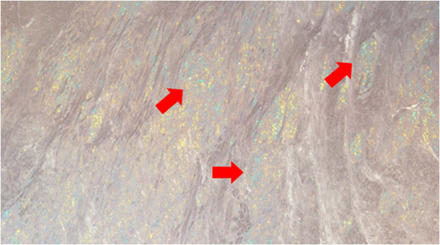
Up until a few years ago, endomyocardial biopsy and subsequent histopathologic analysis with demonstration of pathognomonic apple-green birefringence on Congo red staining (Fig. 1) were the only way to diagnose CA definitively. Tissue biopsies are limited by their inability to ascertain the amyloidogenic protein burden or reveal whether the amyloid deposition process is active. Furthermore, biopsy and staining are operator-dependent, which along with the patchy nature of fibril deposition in some patients, may produce false-negative results (5). A paradigm shift in the diagnostic algorithm of cardiac ATTR toward noninvasive testing occurred with refinement of the techniques for radionuclide scintigraphy with 99mTc-labeled bisphosphonates such as pyrophosphate, 3,3-diphosphono-1,2-propanodicarboxylic acid, and hydroxymethylene diphosphonate. A multicenter study demonstrated that significant cardiac uptake of these 3 bone-avid radiotracers has a 100% specificity and positive predictive value for cardiac ATTR in the absence of monoclonal proteins in serum and urine (6). In fact, the 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America guideline for the management of heart failure now recommends that in patients with a history of electrocardiographic, echocardiographic, or cardiac MRI findings suggestive of CA who do not have detectable serum or urine monoclonal light chains, a [99mTc]Tc-pyrophosphate scan should be performed to confirm the presence of cardiac ATTR (class I) (7). Accordingly, cardiac scintigraphy has now largely replaced the requirement for endomyocardial biopsy to establish the diagnosis in most patients suspected to have cardiac ATTR. What was once considered a rare and incurable disease now has a Food and Drug Administration–approved therapy, with multiple more drugs being investigated with promising results (8,9). Thus, the crucial role of radionuclide scintigraphy to image CA must be underscored.
Endocardial biopsy. Pathognomonic apple-green birefringence (arrows) is seen on Congo red staining of myocardium afflicted with amyloid fibril deposition.
In addition to the growing clinical need for accurate, noninvasive assessment of CA, there is also interest in developing techniques for quantitative estimation of amyloid deposition in the heart. Such estimation may provide important prognostic information and be a means to assess response to therapy. In light of this interest, the aim of this review is to educate the reader on novel imaging tracers for CA. Depending on the molecular target, tracers are divided into amyloid-specific and non–amyloid-specific agents, each with subclassifications (Figs. 2 and 3) (10).
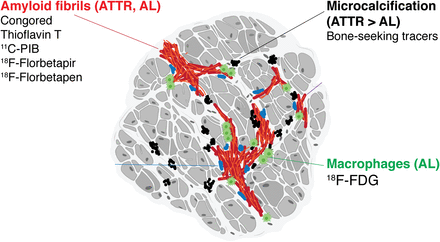
Binding sites for CA radiotracers: Congo red, thioflavin-T, and its analogs 11C-PIB, 18F-florbetapir, and 18F-florbetaben bind to both ATTR and AL amyloid fibrils. 99mTc bone-seeking tracers show avid uptake in ATTR, possibly related to microcalcification. Increased uptake of 18F-FDG in AL is attributed to inflammation and macrophage infiltration. (Reprinted from (23).)
Tracers used for imaging of CA. Nuclear medicine tracers used to image CA can be categorized by those that are non–amyloid-specific (bone-avid tracers, antimyosin, and sympathetic innervation tracers) and amyloid-specific (thioflavin-T derivatives, stilbene derivatives, and protease inhibitors). DPD = 3,3-diphosphono-1,2-propanodicarboxylic acid; HMDP = hydroxymethylene diphosphonate; PYP = pyrophosphate.
NON–AMYLOID-SPECIFIC PROBES
Cardiac Scintigraphy with Bone-Avid Tracers
[99mTc]Tc-pyrophosphate, [99mTc]Tc-3,3-diphosphono-1,2-propanodicarboxylic acid, and [99mTc]Tc-hydroxymethylene diphosphonate are bone-seeking single-photon emitter radioisotopes that are routinely used in clinical practice for the diagnosis of cardiac ATTR. These have been extensively reviewed elsewhere, and this review will be limited to novel tracers. Although current methods of interpretation with the semiquantitative visual grading and quantitative heart–to–contralateral-lung ratio are adequate for diagnosis, they fall short in addressing certain pertinent clinical facets such as reliable detection of early disease, evaluation of response to therapy, assessment of disease progression, and prognostication (11). Though advances in SPECT instrumentation allow for SUV measurement, these are still under investigation, underscoring the need for targeted molecular imaging with novel tracers that may fulfill this clinical gap.
Antimyosin Scintigraphy
Myosin is an intracellular enzyme local to the myocardium and skeletal muscles. Similar to troponin, extracellular spillage of myosin occurs due to damage to these sites. 111In-labeled antimyosin antibodies may be used to detect this extravasation of myosin. Pathologies afflicting the myocardium, such as myocardial infarction, myocarditis, and cardiomyopathies, including CA, are detected by cardiac radiotracer uptake (12–14). A small study found diffuse [111In]In-antimyosin uptake in the left ventricle of all 7 patients with cardiac AL, whereas no uptake was visualized in the control group (15). Despite the lack of specificity for any given cardiomyopathy, the tracer bears mention as a potential risk-stratification and response-to-treatment monitoring tool in patients with previously diagnosed cardiac AL.
Sympathetic Innervation Imaging
[123I]I-meta-iodobenzylguanidine is an analog to norepinephrine and is taken up and stored in sympathetic nerve endings without enzymatic degradation, allowing for objective evaluation of cardiac sympathetic function (16). Cardiac [123I]I-meta-iodobenzylguanidine uptake (heart-to-mediastinum ratio) has been shown to predict clinical outcomes in patients with heart failure (17). Though [123I]I-meta-iodobenzylguanidine does not bind directly with the amyloid fibrils, numerous studies have demonstrated significantly decreased [123I]I-meta-iodobenzylguanidine cardiac uptake in patients with both transthyretin-related familial amyloidosis and cardiac AL (18–20). The decreased uptake is thought to be due to deposition of amyloid fibrils or direct impairment of the autonomic nervous system as seen in these patients. Given that this is an indirect assessment and nonspecific for CA, its clinical utility has remained limited (21).
AMYLOID-SPECIFIC PROBES
Molecular imaging with targeted amyloid-binding radiotracers for PET have the ability to detect all amyloid deposits independently of precursor protein (22,23). In most studies evaluating amyloid deposition in the heart, the radiotracer signal intensity trended higher for AL than for ATTR; however, there was a significant overlap in values, precluding distinction between AL and ATTR (24–26). A prominent advantage of PET tracers is the ability to quantify amyloid burden and thereby track change.
Thioflavin-T Derivatives
Thioflavin-T is a benzothiazole dye that exhibits enhanced fluorescence emission on binding with amyloid fibrils and has become a gold standard for its selective and high-affinity binding to diverse types of amyloid fibrils (27,28).
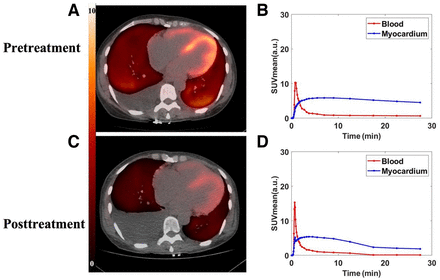
The PET tracer [11C]C-Pittsburgh compound B ([11C]C-PiB) is a radioactive derivative of benzothiazole that binds to any kind of β-amyloid sheet structure (27). Its use in the early detection and prediction of outcomes in Alzheimer dementia led to investigating its utility in detecting CA (29,30). Lee et al. conducted a pilot study that demonstrated the efficacy of [11C]C-PiB PET/CT in the diagnosis of CA (31). [11C]C-PiB PET/CT was positive in 13 of 15 biopsy-proven CA patients, with no false-positive scans. The sensitivity and specificity of [11C]C-PiB PET/CT were comparable to cardiac MRI for assessment of CA. More interestingly, there was a significant difference in [11C]C-PiB PET/CT uptake by the myocardium (assessed by SUV or SUV) between patients who were chemotherapy-naïve and those who had received chemotherapy for cardiac AL (median, 10.4 [range, 1.7–19.9] vs. 2.3 [range, 1.7–3.8]; P = 0.014). This difference raised the possibility of using [11C]C-PiB PET/CT as a surrogate for active light-chain deposition in the myocardium (Fig. 4) (31,32). There have been reports of certain cases of hereditary cardiac ATTR (with substitution of single-amino-acid valine for methionine at position 30 of the transthyretin gene, V-30M) being detected by [11C]C-PiB imaging but not by [99mTc]Tc-3,3-diphosphono-1,2-propanodicarboxylic acid imaging (33,34). A limitation of [11C]C-PiB is its relatively short half-life of 20 min and the requirement for an onsite cyclotron for its production. [18F]F-flutemetamol is a structural analog of [11C]C-PiB with a longer half-life (109 min), thereby obviating an onsite cyclotron, but appropriate imaging protocols for CA have yet to be defined (35–37).
Potential use of novel PET tracers to track disease activity with treatment. These are images of 49-y-old woman with diagnosis of cardiac AL. (A) Pretreatment 11C-PiB PET/CT images show diffuse uptake in both ventricular walls (retention index, 0.231; SUVmean, 5.41). (C) Posttreatment 11C-PiB PET/CT images performed after 9 cycles of chemotherapy with bortezombib, lenalidomide, and dexamethasone show marked reduction in cardiac uptake of tracer (retention index, 0.135; SUVmean, 3.2). (B and D) Time–activity curves before (B) and after (D) treatment. a.u. = arbitrary units. (Reprinted from (32).)
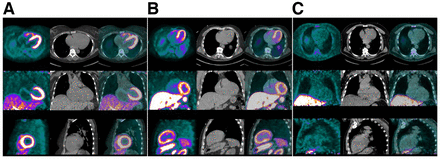
[18F]F-florbetaben has been studied in patients with left ventricular hypertrophy and has demonstrated promise in identifying CA versus controls with heart disease secondary to hypertension (Fig. 5) (24). This study also found that the percentage of [18F]F-florbetaben retention significantly corresponded with biventricular contractile function as measured by longitudinal strain via an inverse curve relationship.
18F-florbetaben in AL CA. Amyloid-specific probes, unlike bone scintigraphy radiotracers, have the added advantage of being able to diagnose AL in addition to cardiac ATTR. Given here are 18F-florbetaben PET (left column in each panel), low-dose CT (middle column), and PET/CT (right column) images of representative AL patient (A), ATTR patient (B), and hypertensive control (C). Of note is diffuse avid 18F-florbetaben myocardial uptake in both AL and ATTR patients but little radiotracer uptake in myocardium of hypertensive control. PET images were windowed to display myocardial boundaries. (Reprinted from (24).)
Stilbene Derivatives
Stilbene is an organic compound whose name is derived from the Greek word stilbo, which means, “I shine.” Certain synthetic fluorinated stilbene derivates display high binding affinity to amyloid fibrils (38).
[18F]F-florbetapir has been approved by the U.S. Food and Drug Administration for imaging β-amyloid protein in the brain, with very high sensitivity for even small foci of deposition (39). A prospectively controlled pilot study of 14 subjects (9 with definite CA and 5 controls without amyloidosis) demonstrated diffuse and uniform biventricular uptake of the radiotracer in all subjects with CA, without discrimination of ATTR and AL subtypes despite a retention index higher in ATTR than in AL. The authors hypothesized that the higher radiotracer uptake in AL subtypes (who have a lower myocardial mass than in ATTR) may be a reflection of disease activity, in addition to amyloid mass. In controls, the [18F]F-florbetapir activity in the blood pool and myocardium peaked early and cleared quickly to very low levels (40). The higher affinity to AL than to ATTR was replicated in vitro using autoradiography, though once again, the 2 subtypes could not be differentiated. The reliable binding to the AL subtype is advantageous over the currently available SPECT bone-avid radiotracers (41). [18F]F-florbetapir also appears to hold promise in the identification of pulmonary involvement in patients with systemic cardiac AL, though the clinical and prognostic implications are still to be determined (42).
Protease Inhibitors
Protease inhibitors interfere with the ability of certain enzymes to break down proteins. The infusion of protease inhibitors (or antiproteases) appears to enhance deposition of β-amyloid in rat brain models (43). [99mTc]Tc-aprotinin is a serum protease inhibitor derived from bovine tissue. Though focused cardiac imaging studies are lacking, Han et al. showed that [99mTc]Tc-aprotinin was taken up by CA-afflicted hearts with clinical evidence of amyloid heart disease, irrespective of subtype (44). Its use is, however, hindered by a limited signal-to-noise ratio and the concern about bovine spongiform encephalopathy, given its origins (10,44).
SUMMARY
There is a growing body of evidence in support of amyloid-specific molecular imaging PET tracers. A metaanalysis of 6 studies including 98 subjects reported a sensitivity and specificity of 95% (45). Although bone-avid scintigraphy agents have minimized the need for invasive endomyocardial biopsies in the diagnosis of cardiac ATTR, PET tracers have the potential to do so for cardiac AL as well. In addition, given that uptake of these tracers in the heart can be quantified, their role in early disease detection, prognostication, monitoring, and assessment of response to therapy is inchoate.
DISCLOSURE
Saurabh Malhotra is on the speakers’ bureaus for Pfizer Inc. and Alnylam and on the advisory boards for Pfizer Inc., Alnylam, and BridgeBio. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What are the recent advancements in nuclear cardiology tracer technology for the management of CA?
PERTINENT FINDINGS: Although bone-avid scintigraphy agents have minimized the need for invasive endomyocardial biopsies in the diagnosis of transthyretin CA, PET tracers have the potential to do so for light-chain CA as well.
IMPLICATIONS FOR PATIENT CARE: Given that uptake of PET tracers in the heart can be quantified, their role in early detection, prognostication, monitoring, and assessment of response to therapy for CA is currently under investigation.
Footnotes
Published online May 16, 2023.
REFERENCES
- Received for publication February 7, 2023.
- Revision received April 14, 2023.