Abstract
We present the molecular PET/CT imaging profile of an interesting case of differentiated thyroid carcinoma that later transformed into TENIS (thyroglobulin elevation and negative iodine scintigraphy) with tyrosine kinase inhibitor–resistant recurrent, aggressive disease. The patient was evaluated to assess somatostatin receptor 2 or PSMA expression to explore whether there might be any effective targeted nuclear therapy. 18F-FDG, 68Ga-DOTATATE, and 68Ga-PSMA-11 PET/CT scans were obtained, all of which revealed tracer avidity in extensive locoregional disease. A large, ill-defined retropharyngeal and retrotracheal soft-tissue mass was seen to be eroding the cricoid cartilage and extending into the tracheal lumen and the left-sided strap muscles. In contrast, there was no definite uptake in the multiple lung nodules that were present bilaterally. The scan findings indicated a differential tumor biology between locoregional and distant metastasis.
TENIS (thyroglobulin elevation and negative iodine scintigraphy) is the major cause of mortality and morbidity in patients with differentiated thyroid carcinoma, as no definitive or effective targeted nuclear therapy is available. In approximately 20%–30% of patients with metastatic or recurrent differentiated thyroid carcinoma, there is evidence of lack of sodium iodide symporter expression, with the disease producing negative findings on radioiodine scintigraphy and, thus, being refractory to radioiodine treatment. Preliminary reports show that such tumors may express somatostatin receptor (SSTR) 2 on their cell surfaces, or there can be prostate-specific membrane antigen (PSMA) overexpression secondary to formation of the tumor neovasculature; PSMA is expressed by the vascular endothelium in a variety of cancers (1). Use of PSMA expression in TENIS to determine potential treatment options was confirmed by recent studies on differentiated thyroid cancer (2–4). Thus, noninvasive imaging for SSTR-2 or PSMA expression in TENIS is being explored in the search for potential definitive or effective treatment options.
CASE REPORT
An 85-y-old man who had papillary thyroid cancer with no extrathyroidal or nodal involvement underwent total thyroidectomy followed, 2 y later, by surgery for locoregional recurrence. After another 3 y, the patient experienced a relapse comprising locoregional inoperable disease, for which he was treated with multiple sessions of oral radioiodine therapy (cumulative dose, 22.94 GBq [620 mCi]). On subsequent follow-up, his 131I whole-body scan was negative, but he had persistent locoregional disease and an elevated thyroglobulin level and, thus, underwent external radiotherapy. Despite thyroxin suppression, his thyroglobulin level persisted in rising, and 18F-FDG PET/CT showed an ill-defined hypermetabolic mass in the tracheoesophageal groove, abutting the trachea anteriorly and the esophagus posteriorly and reaching the paravertebral region at D1/D2. Multiple tiny ametabolic lung nodules (largest, 1.3 cm) were observed bilaterally. The patient was prescribed a tyrosine kinase inhibitor (sorafenib and, later, lenvatinib) and was monitored by 18F-FDG PET/CT. While taking the tyrosine kinase inhibitor, the patient began to have difficulty swallowing and his serum thyroglobulin levels rose to 351.27 ng/mL, despite his having adequate suppression of thyroid-stimulating hormone and being negative for antithyroglobulin antibodies.
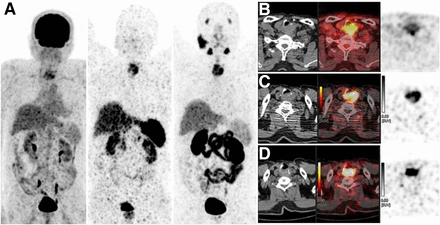
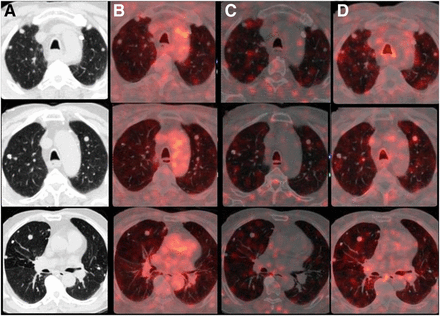
The patient lacked sodium iodide symporter expression. After approval by the institutional ethics committee, he was evaluated by whole-body 18F-FDG, 68Ga-DOTATATE (assessing primarily SSTR2 expression in tumor cells), and 68Ga-PSMA-11 (assessing PSMA expression in neovascular endothelium) PET/CT to determine whether there might be any effective targeted nuclear therapy (Figs. 1 and 2). The 3 scans were compared. There was a locoregional, ill-defined retropharyngeal and retrotracheal soft-tissue mass measuring 5.0 × 3.7 × 3.5 cm eroding the cricoid cartilage, extending anteriorly into the tracheal lumen, abutting the prevertebral fascia posteriorly, involving the strap muscles on the left side, and abutting the common carotid artery. Quantitation of each tracer revealed an 18F-FDG SUVmax of 5.8, an 68Ga-DOTATATE SUVmax of 18.3 and Krenning score of 3 (liver, 14.4; spleen, 36.6), and an 68Ga-PSMA-11 SUVmax of 19.5 and molecular imaging PSMA score of 3 (parotid, 18.3). There were multiple distant lung nodules bilaterally, with the largest measuring 1.3 × 1.1 cm in the right middle lobe; none showed definite uptake on any of the 3 studies (Figs. 1 and 2). Palliative 177Lu-PSMA-617 therapy began. A posttherapy scan showed adequate uptake in the upper mediastinal soft tissue and was to be followed up by another scan after 2 mo. Relief of locoregional pain and greater ease in swallowing indirectly improved the quality of life within 1 mo after therapy. This case demonstrated—in the same individual—differential tumor biology between tracer-positive extensive locoregional disease (18F-FDG, 68Ga-PSMA-11, and 68Ga-DOTATATE with a high molecular imaging PSMA score and a high Krenning score) and tracer-negative distant lung metastases.
Images showing soft tissue in retropharyngeal space with tracheal invasion and posteriorly abutting prevertebral fascia. (A) Maximum-intensity-projection anterior PET images: 18F-FDG, 68Ga-DOTATATE, and 68Ga-PSMA-11 (from left to right). (B–D) Axial CT, PET/CT, and PET images (from left to right) obtained with 18F-FDG (B), 68Ga-DOTATATE (C), and 68Ga-PSMA (D).
Axial lung-window images at 3 different levels showing non–tracer-avid multiple bilateral lung nodules: CT (A), 18F-FDG PET/CT (B), 68Ga-DOTATATE PET/CT (C), and 68Ga-PSMA PET/CT (D).
DISCUSSION
Silberstein stated that physicians caring for patients with TENIS syndrome are urged to enter them into clinical therapeutic studies whenever possible (5). TENIS tumors show variable expression of SSTR-2 on their cell surfaces (6–8) or PSMA in the neovasculature of the apical surface of endothelial cells (9). 68Ga-DOTATOC or 68Ga-DOTATATE PET/CT can be used for visualization of SSTR-2–expressing lesions. However, not all patients with TENIS lesions express SSTR-2 (6–8). Use of PSMA expression in TENIS as a way to determine potential treatment options was confirmed by studies on differentiated thyroid cancer (1,10,11). PSMA, representing a marker of neovasculature formation expressed by differentiated thyroid carcinoma, has been proposed to contribute to the prediction of tumor aggressiveness and patient outcome (4). One reason for lack of uptake by any of the 3 tracers in the lung metastases could also be the limited PET spatial resolution and the partial-volume effect, particularly considering how small most lesions were. In TENIS, visual evaluation using the Krenning score (68Ga-DOTATATE) and the molecular imaging PSMA (68Ga-PSMA-11) score is a promising approach in exploring tumor biology in metastatic disease and can create the possibility of targeted therapy with177Lu-DOTATATE and 177Lu-PSMA, depending on tracer avidity on 68Ga-DOTATATE and 68Ga-PSMA-11 PET/CT (12). A high uptake (such as in this case, with a molecular imaging PSMA score of 3 and a Krenning score of 3 for uptake in aggressive locoregional disease) potentially qualifies the patient for targeted SSTR- or PSMA-based therapies as promising options in the absence of other treatments.
CONCLUSION
The present case highlights the differential tumor biology between positive extensive locoregional disease and negative distant lung metastasis, explored by molecular imaging with PET/CT.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Dec. 6, 2022.
REFERENCES
- Received for publication October 29, 2021.
- Revision received November 1, 2021.