Abstract
Bone scintigraphy is one of the most common nuclear medicine tests. Previous work investigated the effectiveness of an asymmetric window (ASW) for planar bone scintigraphy using simulation and phantom data. Phantom studies concluded that the ASW improved both the resolution and the contrast-to-noise ratio when imaging objects with high scatter. The aim of this study was to confirm this improvement increased image quality in patients. This study also investigated whether the differences between a symmetric window (SW) and an ASW depended on body mass index. Methods: Fifty-eight patients had 2 scans: a standard scan using an SW of 140 keV ± 10% and a scan using an ASW of 140 keV + 10% and − 7.5%. Three readers independently compared the 2 image sets and scored them using a 5-score scale (ranging from 1 = ASW better [clinically important] to 5 = SW better [clinically important]). Scores from all radiologists were pooled and analyzed statistically. A P value of less than 0.05 was considered statistically significant. Results: In 93 cases (53%), the readers scored the ASW images better than the SW images. In 5 cases (3%), the ASW images were preferred, with the difference considered clinically important; there were no cases in which the SW was similarly preferred. For the sign test, we determined whether the total of 93 scores of 1 or 2 (ASW preferred) was significantly different from the 15 scores of 4 or 5 (SW preferred). The P value was less than 0.00001, demonstrating that the difference was significant. Conclusion: In patients undergoing bone scintigraphy, ASW provided an improvement in image quality that in some cases was judged clinically important.
Bone scintigraphy is one of the most common nuclear medicine procedures performed in the United Kingdom and in the United States, representing 29.6% of nuclear medicine procedures in 2003/2004 (the most recently available national survey) and 17% in 2011 (1,2). Although bone scintigraphy is considered to be one of the most reliable nuclear medicine examinations (3), the detection of lesions is affected by image quality, which depends on several different variables, such as age (4,5), time between dose and scan (6,7), radiopharmaceutical (8), distance between the patient and the collimator (3), and body mass (9).
Scatter, an important process in nuclear medicine imaging, usually refers to Compton scattering. A γ-ray interacts with matter (e.g., the patient), decreasing the energy of the γ-ray and deflecting it from its original path (10). Scattered photons are one of the main defects that degrade the quality of nuclear medicine images and sometimes represent more than half the total counts in clinical imaging (11,12). Scatter deflects photons, with a concomitant loss of energy leading to blurring, loss of image contrast, and, consequently, poor image quality (13,14).
Detector technology has advanced rapidly, but there are still profound challenges in diagnostic imaging with regard to scatter radiation, particularly in obese patients. An increased body mass attenuates photons by absorbing and scattering them within the soft tissue, leading to a reduced signal-to-noise ratio, increased scatter, and nondiagnostic results (9,15).
Most nuclear medicine departments apply predefined protocols designed to exclude scattered photons through use of a suitable energy window, which is an accepted trade-off between minimizing the acceptance of scattered photons and maximizing the acceptance of unscattered radiation (16).
The photopeak is the peak formed when an incident γ-ray is completely absorbed in the crystal because of the photoelectric effect (17). The event detection necessary to produce an image occurs in an energy window around the photopeak energy, which is typically a 15% or 20% symmetric window (SW) centered over the 140-keV photopeak of 99mTc. This SW is equivalent to a window spanning 130–151 keV and 126–154 keV, respectively (18,19). An asymmetric window (ASW) is produced when the photopeak energy is not at the center of the window because the energy window has been shifted to the right side on the spectrum (20). An ASW can be used in practice to avoid lower-energy photons and to minimize the amount of scatter that is recorded (21). The number of counts collected is reduced, thus elevating noise, and scatter is still present but at a reduced level (22). Use of an ASW must be supported by a physicist who can help in determining the limits of asymmetry (18,19). In this study, an ASW of −7.5% and +10% over the 140-keV photopeak of 99mTc was investigated, which is equivalent to a window spanning 129.5–154 keV.
Although attempts have been made to evaluate the effect of an ASW on contrast resolution (23), scatter and attenuation correction (24), and flood-field uniformity (25), there are no data about the use of an ASW to improve image quality in whole-body bone scans obtained on contemporary equipment. This study aimed to transfer the results of our phantom work to patients to confirm that image quality in whole-body bone scans is improved by using an ASW. Associations were examined between image quality scoring and body mass index (BMI).
MATERIALS AND METHODS
Patient Population
The study included 36 male and 22 female patients with an age range of 31–87 y who were seen from January 2014 to February 2016. Among the 58 patients, most presented with prostate cancer (n = 32) or breast cancer (n = 12). Patient weight averaged 79.8 kg.
All patients underwent SW whole-body imaging first, followed by an ASW whole-body scan.
The patients were categorized into 4 groups according to BMI as defined by the National Health System: underweight (BMI < 18.5 kg/m2), healthy weight (BMI = 18.5–24.9 kg/m2), overweight (BMI = 25–29.9 kg/m2), and obese (BMI = 30–39.9 kg/m2).
Approval for the study was obtained from the Bristol Research Ethics Centre (institutional review board equivalent), and all subjects gave written informed consent. All procedures were in accordance with the 1964 Helsinki declaration.
The datasets generated during or analyzed during the study are available from the corresponding author on reasonable request.
Imaging Technique
The study was done using a dual-head Infinia Hawkeye γ-camera (GE Healthcare), equipped with low-energy, high-resolution collimators and capable of simultaneous anterior and posterior acquisition. Quality assurance testing was conducted daily before scanning. A new uniformity map was acquired for the ASW acquisition.
A SW whole-body scan was obtained 2–3 h after injection of 546–640 MBq of 99mTc-hydroxydiphosphonate as per the British Nuclear Medicine Society guidelines for bone scintigraphy (26). Patients were invited to empty the bladder before the first part of the acquisition. Directly after the SW whole-body scan, an ASW whole-body scan was performed.
The SW acquisition ranged from the top of the head to the bottom of the feet, and the ASW acquisition ranged from the top of the head to the knees. The field of view was shorter for the ASW than for the SW to reduce the acquisition time, as well as to avoid patient movement and, consequently, degradation of image quality.
The 2 sets of images were obtained in both anterior and posterior projections, with the patients supine and a hook-and-loop strap used to help them keep their arms by their sides. A pillow to support the head was used for the duration of the examination. Scan speed was 10 cm/min, exposure time was 240 s, matrix size was 256 × 1,024, and zoom was 1.0. The energy window was defined relative to the photon energy peak of 99mTc (140 keV) for both scans. The window width was set at −10% and +10% (126–154 keV) for the SW scan and −7.5% and +10% (129.5–154 keV) for the ASW scan, as illustrated in Figure 1.
γ-spectrum and photopeak parameters of 99mTc isotope using SW (A) and ASW (B) recorded during whole-body bone scintigraphy. Screenshots are from GE Healthcare Discovery NM/CT 670 workstation.
After both scans were performed, the patients’ weight and height were measured.
Image Assessment
To assess the impact of an ASW on clinical images, 3 experienced radiologists with a specialist interest in nuclear medicine who were unaware of the clinical information examined the scans using a dedicated image analysis workstation (Xeleris; GE Healthcare). A coding system was used to anonymize the patient data before image analysis.
The 2 whole-body images were displayed on the same screen and using the same color scale. Briefly, the visual evaluation consisted of comparing the intensity of abnormal uptake in the SW image with that in the ASW image and scoring the differences as in Table 1. BMI was not compared with image outcome
Five-Point Scoring System
Statistics Assessment
We tested 2 hypotheses: that a preference for SW or ASW is equally likely (the null hypothesis) and that a preference for either SW or ASW is more likely.
The sign test was used for statistical analyses. In this test, a score of 1 or 2 indicated that ASW was preferred, and these 2 scores were added together to determine the total number for which ASW was preferred (N_A). A score of 4 or 5 indicated that SW was preferred; likewise, these 2 scores were added together to determine the total number for which SW was preferred (N_S). The sign test is a test of whether N_A is drawn from a binomial distribution ([N_A ∼ bin(N_tot, q)], with N_tot = N_A + N_S and q = 0.5). An α-value of 0.05 was used to determine whether a P value indicated a significant result. The calculations were performed using a freely available online binomial calculator (http://stattrek.com).
RESULTS
In total, 58 patients were enrolled and 116 images analyzed: 58 SW and 58 ASW. The 3 independent radiologists scored the images according to the 5-point scoring system, resulting in a total of 174 scores.
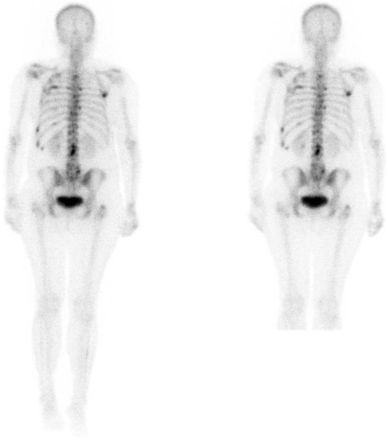
Example images from the SW and ASW acquisitions are shown in Figures 2 and 3.
SW (left) and ASW (right) whole-body bone scintigraphy in breast cancer patient with coexistence of bone metastases. Widespread foci of abnormal tracer activity are seen within skull, thoracic spine, ribs, shoulders, lumbar spine, and proximal femora in keeping with widespread bony metastatic disease. All readers rated ASW as preferred but not clinically important.
SW (left) and ASW (right) whole-body bone scintigraphy in breast cancer patient. Thoracolumbar junction region shows minor increased uptake that appears to correlate with degenerative change seen on radiography at T12–L1. One reader rated ASW as preferred but not clinically important. Another reader rated SW as preferred and clinically important because it potentially improves assessment of lumbar spine. Third reader rated neither as preferred.
Table 2 and Figure 4 show the results of the image comparison. The data are ordinal, allowing calculation of median. There were 5 possible scores, with the middle score (3) representing no preference for either SW or ASW; most sets of images were assigned this score. Scores of 1 and 2 represented a preference for ASW, and scores of 4 and 5 represented a preference for SW; it is these scores that we analyzed using the sign test. In total, 93 scores were less than 3, and 15 scores (8.6%) were more than 3. Use of the sign test to compare an N_A of 93 with an N_S of 15 gave a P value of less than 0.000002; we therefore rejected the null hypothesis that ASW and SW are equally likely to be preferred.
Percentages of Comparison Scores for 3 Independent Readers
Bar graph comparing the pairs of images for all 58 patients. Results are expressed as radiologists 1, 2, and 3 against different imaging scores.
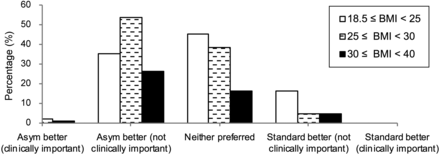
Mean BMI was 27.7 kg/m2. Figure 5 presents the imaging scores analyzed by patient BMI. Within the group that had scores of less than 3, the proportion of obese patients was similar to that of nonobese patients. A score of less than 3 was reported for 70% of healthy-weight patients and 92% of overweight patients, versus 85% of obese patients. No significant differences across BMI categories were observed for the different imaging scores.
Bar graph comparing the pairs of images according to patient BMI. Results are expressed as BMI intervals against different imaging scores.
DISCUSSION
In this study, we evaluated the effectiveness of using an ASW in planar bone scans. To our knowledge, this study was the first to explore the impact of an ASW on image quality in planar bone scans obtained on contemporary equipment.
The imaging parameters may be further adapted to the clinical indication by decreasing the time between injection and imaging, reducing the acquisition time, or increasing the administered activity (14). The work presented here did not consider these factors.
As Asgari et al. (18) indicated, scatter fraction seemed to be lower with an ASW than with an SW in 99mTc and 153Sm imaging of a Solid Water (Sun Nuclear Corp.) slab phantom and Teflon (DuPont) bone phantoms. In an earlier study (23), Collier et al. found that contrast resolution was significantly improved by switching to an ASW in anterior views of the abdomen and lumbar spine 3 h after the injection of 740 MBq of 99mTc-methylene diphosphonate.
With ASW, the energy window is smaller and more counts will be rejected. To maintain the number of counts in the final image, one would have to increase the injected activity or the scanning time. However, in this case we used a fixed standard couch-speed for both the ASW and the SW scans. The ASW images are still preferred despite the reduced overall counts. Increasing the acquisition time may increase the quality of images still further.
Shifting the 99mTc window to the right has been shown to be effective in reducing scatter. In most cases in which a preference was expressed, an ASW was preferred. We did not randomize the order of the scans; we performed SW imaging first because that is the standard care and patients should be able to decline the second scan at any time. It is therefore possible that the ASW was preferred not because of its asymmetry but because of the systematic timing difference, leading to differences in, for example, uptake and washout. However, it is well established that whole-body images are usually acquired 2–5 h after injection. We therefore presume that the asymmetry is more likely to be the reason for the expressed preference of the ASW.
Obesity is well known to present challenges in various imaging modalities. Problems associated with dose to be administered, image acquisition time, and image noise are still being discussed in the literature. There are no clear conclusions about how we can overcome these challenges, but efforts have been made to investigate weight-based dosing and imaging time (9,15). Our study found similar results in the different BMI groups, possibly because of the limited number of patients included. No comparisons were made between men and women or between different clinical indications. Moreover, the effects of obesity on image quality were not directly analyzed. An alternative approach would be to monitor differences in injected activity between the normal, overweight, and obese groups.
Our study had some limitations. Although we found a highly significant overall result, we did not find any statistically significant effect related to BMI. We suspect that a larger number of patients would be needed to demonstrate such an effect.
We did not undertake quantitative measurements of signal-to-noise ratio; such measurements might have disentangled the effect of uptake and washout.
We relied on qualitative comparison scores, not quantification of contrast as has been done in phantom work. This choice was because we are interested in clinical practice and the utility of the images to radiologists.
Finally, we decided on a shorter field of view for ASW to avoid motion. However, this shorter field of view should not be excluded as a possible confounder, because it prevented the scorers from identifying lesions in lower limbs and also made blinding the scorers impossible.
Nonetheless, after many years of clinical evaluation, bone scintigraphy continues to have a significant impact on patient management, mainly in the evaluation of metastatic disease. There are no comprehensive guidelines on an ideal strategy for using an ASW, but we have proved the feasibility of our technique in whole-body scans that can be used in clinical practice. We expect that this technique will be clinically applied and will have an impact on lesion detection.
CONCLUSION
Incorporation of the ASW into the acquisition protocol for bone scintigraphy presents an important tool in scan optimization. We expect that this technique will be clinically used and have an impact on lesion detection. Our study demonstrated that using an ASW was preferred by radiologists interpreting bone scintigraphy, in some cases in a way that was judged to be clinically important. Further research is necessary to determine the influence of using an ASW in obese patients.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We gratefully acknowledge the assistance of our nuclear medicine technologists with data acquisition.
Footnotes
Published online Oct. 11, 2019.
REFERENCES
- Received for publication July 8, 2019.
- Accepted for publication August 30, 2019.