Abstract
There has been a significant increase in cardiac radionuclide imaging over the past decade, leading to a corresponding increase in scrutiny from Federal and private health plans questioning the necessity of these tests. In response to efforts by third-party payers to limit all types of cardiovascular imaging studies, the American College of Cardiology Foundation, in conjunction with other professional societies, developed appropriate use criteria. The goal of this article is to explain how the criteria were created and define the 3 categories of indications: appropriate, inappropriate, and uncertain. Tips for using appropriate use criteria and tables, including a definition of several key terms technologists should be familiar with, will be provided. In addition, reimbursement, benchmark data, and practical considerations for implementation will be discussed. Finally, several tools to aid in calculating appropriateness are suggested. With a basic understanding, the appropriate use criteria are relatively easy to apply. It is important for facilities to begin to voluntarily incorporate them into their practice and document levels of appropriateness now as payers are developing 2 trends that are not favorable for nuclear cardiology: preauthorization and denial of payment for inappropriate studies.
Most technologists are aware there has been a significant increase in cardiovascular imaging—to be specific, myocardial perfusion imaging—over the past decade (1). This has led to a corresponding increase in scrutiny from payers questioning the necessity of these tests. And while diagnostic imaging provides many benefits to patients and is critical in the delivery of quality health care, the cost ramifications associated with this increased imaging must be acknowledged and addressed.
Numerous third-party payers have devised methods of use management with the aim of decreasing the volume of studies performed and thus driving down costs (2). Examples of such methods include prior authorization, prenotification, denial of claims, and the increased involvement of radiology benefits management companies. None of these methods, however, are founded on quality and good patient outcomes.
In response to efforts by third-party payers to limit imaging studies, the American College of Cardiology Foundation (ACCF), in conjunction with other professional societies, developed appropriate use criteria (AUC) for the most commonly performed cardiovascular imaging studies (3–6).
The main objective of AUC is to identify patients who will most appropriately benefit from a procedure and those who will not, thus resulting in a more effective and equitable allocation of health-care resources. AUC have been developed for myocardial perfusion imaging, including SPECT and PET, cardiovascular CT, cardiovascular magnetic resonance, and echocardiography.
The AUC help guide physician decision making by providing objective, evidence-based guidance regarding the use of cardiovascular imaging in the most common clinical scenarios.
Ensuring that the right patient receives the right test at the right time is the ultimate goal. The decision of when and how often to do an imaging study is based on 3 factors: scientific data, the patient’s clinical condition, and the physician’s judgment. It is expected that AUC will help to minimize inappropriate use, increase use in specific high-risk populations such as diabetic patients or patients with heart failure, improve image quality, and facilitate appropriate reimbursement. Although the criteria are intended to provide guidance in decision making, they are not intended to replace sound clinical judgment and practice experience.
HOW THE AUC WERE DEVELOPED
Before the development of AUC, test selection was steered by clinical guidelines and performance measures. These documents are often complex and unwieldy, making it difficult for clinicians to know when to perform a specific study. Beginning in 2005, the first set of appropriateness criteria were developed by focusing on reasonable use for a given procedure (7).
To build the criteria, a panel of experts performed an extensive review of the literature including guidelines and defined a group of typical clinical scenarios. Using the methodology of the RAND Corporation and the University of California at Los Angeles, these scenarios were independently assessed and scored by a separate panel of 13–15 members consisting of imaging experts, clinicians, and public health experts. After this initial independent evaluation, a face-to-face consensus conference was held and the scoring reviewed and an attempt made to reach consensus. Approximately 2 wk later, the experts independently rescored the criteria and this constituted the final scoring for level of appropriateness (8).
One of the most important steps in developing the AUC was agreement during the face-to-face meeting by the experts. The definition had to consider specific test performance characteristics for a specific clinical indication, the possible negative consequences of imaging, the impact of cost, and realization of how the test results might lead to care that could improve the patient’s chances for better survival or improved health status. With these considerations in mind, the following definition of appropriate cardiovascular imaging was used (7):
“An appropriate imaging study is one in which the expected incremental information, combined with clinical judgment, exceeds the expected negative consequences by a sufficiently wide margin for a specific indication that the procedure is generally considered acceptable care and a reasonable approach for the indication.”
The negative consequences referred to in this quotation include the risks of the procedure radiation or contrast exposure and the downstream impact of poor test performance such as a delay in diagnosis (false-negatives) or an inappropriate diagnosis (false-positives).
The goal was to determine whether, based on scientific evidence, an experienced physician in a specific clinical situation would conclude that performing the imaging study is reasonable when providing quality, clinical care.
ABOUT THE CRITERIA
The appropriateness criteria are scored 1–9, with 1 being inappropriate and 9 being the most appropriate, and this results in 3 categories: inappropriate (1–3), uncertain (4–6), and appropriate (7–9). Ratings from 7 to 9 indicate use of radionuclide imaging that is appropriate, generally acceptable, and a reasonable approach. Ratings from 1 to 3 signify a radionuclide imaging study that is inappropriate for that indication and generally not acceptable or not a reasonable approach.
Scenarios that rated from 4 to 6 were considered neither appropriate nor inappropriate but uncertain. For a specific uncertain indication, the test may be acceptable and a reasonable approach (Fig. 1).
Ranking of indications.
The inaugural set of AUC for SPECT myocardial perfusion imaging was published in 2005 by the ACCF and the American Society of Nuclear Cardiology (9). Of 52 clinical scenarios, 27 were rated as appropriate, 12 were rated as uncertain, and 13 were rated as inappropriate.
In 2009, the AUC were refined for radionuclide imaging, including cardiac PET, and published again, but this time with the involvement and endorsement of many additional professional societies. (Professional Societies supporting the 2009 radionuclide imaging AUC include the ACCF Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Nuclear Medicine, and the American College of Emergency Physicians.)
The 2009 AUC comprise 67 common indications, 33 of which were appropriate, 9 uncertain, and 25 inappropriate. New indications were added to the 2009 criteria for heart failure, atrial fibrillation, ventricular tachycardia, syncope with high–coronary heart disease risk, and selected elevated troponins.
An example of an appropriate indication for radionuclide imaging would be testing done on a patient with known coronary artery disease by prior cardiac catheterization with worsening symptoms. The criteria affirm that performing radionuclide imaging on a patient with this clinical scenario is a reasonable approach and likely to improve the patient’s clinical outcome.
According to the criteria, an example of an inappropriate indication is performing testing on a patient without symptoms and less than a year removed from a coronary revascularization procedure. Radionuclide imaging for this reason is generally not acceptable. Inappropriate use of cardiovascular imaging has the potential to be harmful to patients and result in unwarranted radiation and costs to the health-care system.
An example of an uncertain clinical indication is imaging of a patient with a low to intermediate risk of coronary heart disease with a coronary calcium Agatston score between 100 and 400. It is important to stress that an uncertain designation for an indication means there is insufficient evidence or experience with the imaging study or detailed patient characteristics for the indication to definitively be categorized.
An uncertain indication implies that further research or patient information is needed. It does not mean the imaging test should not be performed in that particular situation or that there is no evidence of benefit.
USING THE CRITERIA OR TABLES
The 2009 criteria are sorted into 8 tables (Table 1). The indications are categorized on the basis of detection of coronary artery disease in symptomatic patients, detection of coronary artery disease in asymptomatic patients, risk assessment, assessment of viability, and evaluation of ventricular function.
2009 AUC Tables (by Indication)
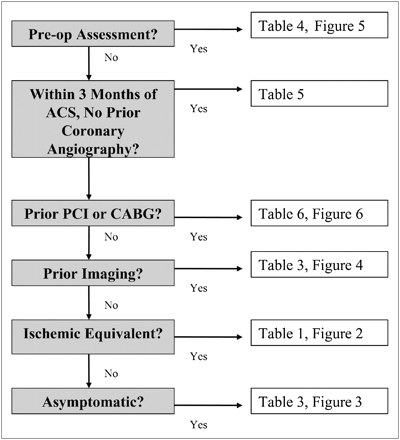
These logical groups of similar indications make it easy to apply the criteria to real patient scenarios. To facilitate use of the criteria, a hierarchy of potential test ordering was created. An algorithm points users to the correct table based on the patient’s clinical presentation (Fig. 2).
Hierarchy of potential test ordering. ACS = acute coronary syndrome; PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft. Reprinted with permission of (3).
The appropriateness of the indication can easily be determined by answering “yes” or “no” to 6 simple questions, going to the applicable table, and then selecting an indication based on the patient’s pertinent medical condition.
The first question in the algorithm is whether the patient is being studied as part of a preoperative assessment. The appropriateness determination for preoperative imaging follows the American College of Cardiology (ACC)/American Heart Association 2007 Perioperative Guidelines (10). If the patient is being studied for preoperative assessment, the indications in Table 4 are applicable. Surgical risk, clinical risk factors, functional capacity, and patient symptoms are also considered in further narrowing to the correct indication.
If the patient is not being studied for preoperative assessment, the next question is whether the patient is within 3 mo of an acute coronary syndrome. If the patient is within 3 mo of an acute coronary syndrome, the indication is determined through use of Table 5 along with the patient’s symptoms and whether the patient had a prior coronary angiogram.
If the patient is not within 3 mo of an acute coronary syndrome but is being evaluated after percutaneous coronary intervention or coronary artery bypass graft surgery, the clinical indication is determined by evaluation of Table 6. The patient’s symptoms, success of revascularization, and time of last test are used to determine the indication. Radionuclide imaging is appropriate when the patient has new symptoms after revascularization. However, if there are no new symptoms, imaging appropriateness depends on the interval since percutaneous coronary intervention or coronary artery bypass graft surgery.
The next question is whether the patient has undergone prior testing such as exercise stress testing, imaging, coronary angiography, or coronary calcium scoring. The type of prior testing, length of time since testing, and whether the patient is symptomatic are considered in Table 3. The criteria suggest that it is inappropriate to repeat stress imaging within 2 y unless the patient has new or worsening symptoms.
The final questions in the algorithm relate to whether the patient has symptoms. If the patient has an ischemic equivalent, then Table 1 is used to determine appropriateness. Key in the evaluation of symptomatic patients is the patient’s pretest probability of coronary artery disease, ability to exercise, and electrocardiogram (ECG) interpretability.
If the patient is asymptomatic, Table 2 is used. To determine appropriateness in an asymptomatic patient, the patient’s coronary heart disease risk using the Framingham risk score and interpretability of the ECG must be considered. In addition, other factors such as the presence of heart failure, syncope, atrial fibrillation, or ventricular tachycardia are important and radionuclide imaging may be justified.
Pretest Probability of CAD by Age, Sex, and Symptoms
Most Frequent Inappropriate Indications
Of the remaining 2 tables, Table 7 has only one clinical indication and is used for the assessment of viability in patients eligible for revascularization with severe left ventricular dysfunction. Table 8 is used in the evaluation of left ventricular function by equilibrium or first-pass radionuclide angiography.
IMPORTANT DEFINITIONS
There are many definitions and processes within the criteria with which technologists must be familiar to correctly apply the criteria to a given clinical scenario. These include ischemic equivalent, coronary heart disease risk, anginal classification, and pretest probability of coronary heart disease for symptomatic patients.
Ischemic equivalent, such as chest pain syndrome, anginal equivalent, or ischemic ECG abnormalities, refers to any clinical findings the physician believes are consistent with obstructive coronary artery disease. Chest pain, chest tightness, burning, shoulder pain, palpitations, jaw pain, new ECG abnormalities, and shortness of breath are examples of patient descriptors of ischemic equivalent.
Coronary heart disease risk is classified into 3 levels of risk, with absolute risk defined as the probability of developing coronary heart disease, including myocardial infarction, or coronary heart disease death over a given period (11). Low coronary heart disease risk is risk that is below average level and correlates with a 10-y absolute coronary heart disease risk of less than 10%.
Moderate coronary heart disease risk is risk that is average or above average and correlates with a 10-y absolute coronary heart disease risk of 10%–20%. High coronary heart disease risk is defined as the presence of diabetes mellitus in a patient 40 y or older, peripheral arterial disease or other coronary heart risk equivalents, or a 10-y absolute coronary heart disease risk of greater than 20%.
Angina can be described as typical or definite, atypical or probable, or nonanginal. Typical—or definite—angina is substernal chest pain or discomfort provoked by exertion or emotional stress and relieved by rest or nitroglycerin. Atypical—or probable—angina is chest pain or discomfort lacking one of the characteristics of typical angina. And finally, nonanginal chest pain is chest pain or discomfort meeting one or none of the typical angina characteristics.
The pretest probability of coronary artery disease for symptomatic patients can be determined by several methods. The method assumed for the AUC is the ACC/AHA 2002 Guideline Update for Exercise Testing (12) and the ACC/AHA 2002 Guideline Update for Management of Patients with Chronic Stable Angina (13). Using the previously listed definitions of angina and Table 2, the pretest probability of coronary artery disease can then be determined.
A very low pretest probability is less than a 5% likelihood of coronary artery disease. A low pretest probability is between a 5% and 10% likelihood of coronary artery disease. An intermediate pretest probability is between a 10% and 90% likelihood of coronary artery disease. And a high pretest probability is a greater than 90% likelihood of coronary artery disease.
REIMBURSEMENT
In the future, it is hoped that appropriateness criteria will be used to facilitate reimbursement and that they will become the foundation on which third-party payers will base coverage decisions. It is expected that appropriate indications will receive reimbursement. By contrast, inappropriate indications will likely require additional documentation to justify payment because of unique circumstances or the clinical profile of the patient.
An uncertain rating implies possible appropriateness. The appropriateness criteria firmly state that an uncertain indication should be reimbursed by payers in some patients if there is sufficient clinical evidence to perform the study.
BENCHMARKS
Incorporating the measurement of appropriateness criteria into clinical practice allows physicians to assess current practice patterns and identify areas needing improvement. A review of literature published since 2005 when the first AUC were published consistently demonstrates that approximately 10%–14% of all studies performed are for inappropriate indications (14,15).
A recently published pilot study done in conjunction by United Healthcare and the ACCF used the 2005 Appropriateness Criteria to evaluate almost 6,000 patients at multiple practice sites. The results of that study demonstrated an overall inappropriateness rate of 14% (16). The study further examined the specific inappropriate indications and their percentage of the overall total studies. It was noted that 5 indications comprised over 92% of inappropriate imaging (Table 3). The most frequent inappropriate indication, encompassing 44.5% of the inappropriate indications, was for the detection of coronary artery disease in asymptomatic patients with low coronary heart disease risk. This represents 6% of the overall total studies. A facility that focuses on decreasing just the studies performed for this indication could significantly reduce the number of inappropriate studies performed.
PRACTICAL CONSIDERATIONS
There are several practical considerations to be considered before a facility incorporates the AUC into daily practice. First and foremost, what is the best time to apply appropriateness criteria to studies referred to the laboratory? Ideally, they should be applied at the time of scheduling and not at the time of service. It is inconvenient for the patient and not cost-effective to cancel a study after a patient arrives at the facility expecting the study to be performed.
Another consideration is concern about the reaction from the referring physician if the study is not performed. Currently, most facilities will perform the study even if it is not appropriate, but in the future, third-party payers may not reimburse for an inappropriate study. Each facility will need to evaluate and plan how to best implement AUC and then provide feedback to referring physicians.
TOOLS
There are several free, easy-to-use tools available to help facilities learn about and measure appropriate use. One of the most robust tools was created by the ACC and is part of its Formation of Optimal Cardiovascular Use Strategies (FOCUS) Innovation Community. Located within the performance improvement module is a program that calculates and tracks appropriate use. ACC membership is not required to access the program, but facilities are required to register. FOCUS can be accessed at www.cardiosource.org/Science-And-Quality/Quality-Programs/Imaging-in-FOCUS.aspx.
Astellas Pharma US, Inc., released a free application in the fall of 2010 to help guide facilities through the process of determining whether cardiac radionuclide imaging is appropriate. The application is currently available for the iPhone (Apple Inc.), Android (Google Inc.), and Blackberry. In addition, there is a Web-based program. The Astellas application can be accessed at www.astellasapps.com.
Skyscape, Inc., a company specializing in mobile medical information, has a tool available based on the 2005 criteria and is developing a new tool to calculate appropriate use based on the 2009 version of the guideline. The new tool, expected to be available on its Web site soon, can be accessed at www.skyscape.com.
CONCLUSION
With a little knowledge, the AUC are easy to use. They are becoming more widely accepted in the health-care arena and, it is hoped, will begin to play an increasingly prominent role in use management. Moving forward, health-care professionals providing imaging services must take responsibility for the imaging studies performed at their facility even if not ordered by staff within the facility. Although quality imaging is extremely important, studies must also be appropriate. As a field, we must come up with methods to assess and document levels of appropriateness in practice to continue providing quality health care.
Acknowledgments
We greatly appreciate the editorial assistance provided by Jeffrey P. Rhodes. No potential conflict of interest relevant to this article was reported.
Footnotes
Published online May 2, 2012.
↵* NOTE: FOR CE CREDIT, YOU CAN ACCESS THIS ACTIVITY THROUGH THE SNM WEB SITE (http://www.snm.org/ce_online) THROUGH June 2014.
REFERENCES
- Received for publication August 27, 2011.
- Accepted for publication December 7, 2011.