Abstract
Patient motion during myocardial perfusion SPECT is a common source of errors. The extent and severity of motion artifacts have been described for filtered backprojection (FBP) reconstruction. In recent years, iterative reconstruction has been used increasingly in reconstruction of myocardial perfusion SPECT images and has been shown to be more accurate than FBP even in cases of incomplete datasets. This study evaluated the effect of iterative reconstruction on the extent and severity of motion artifacts. Methods: Six normal, motion-free, and nongated 99mTc myocardial perfusion SPECT scans were selected, and simulated motion of 3 pixels was applied to the early, middle, and late phases of acquisition in 2 types of movement, returning and nonreturning. The images were acquired by a single-head γ-camera in 32 steps at 30 s per step and in a 180° arc from right anterior oblique to left posterior oblique. All original and shifted images were reconstructed using FBP and ordered-subset expectation maximization (OSEM) techniques and interpreted by 2 nuclear medicine specialists qualitatively and semiquantitatively (using 17 segments and a 5-point scoring system). Results: Overall, 68.1% and 70.8% of shifted images were categorized as definitely abnormal in the FBP and OSEM reconstructions, respectively (P > 0.5). The mean summed score was 11.9 (±5.7) and 11.3 (±5.2) for nonreturning shifted images (P = 0.13) and 5.2 (±2.4) and 3.9 (±2.0) for returning shifted images (P < 0.001) in the OSEM and FBP reconstructions, respectively. The incidence of defects in different myocardial segments was similar with the 2 reconstruction methods. The summed score was higher with shifting in the middle phase of acquisition than in the late or early phase. Conclusion: Our study showed that the incidence of abnormal findings and the location of defects were not different between the 2 reconstruction types; however, with semiquantitative assessment, the severity of defects increased with OSEM reconstruction. Although OSEM reconstruction has been reported to be more tolerant to missing data than is FBP reconstruction, our study showed that OSEM reconstruction may be less tolerant to motion artifacts than is FBP reconstruction.
Patient or organ motion during myocardial perfusion SPECT is believed to affect as many as 10%−20% of all cardiac SPECT studies and is a well-recognized source of errors in scan interpretation (1–4). The pattern and severity of motion artifacts have been studied for actual or simulated motion during SPECT acquisitions using single-head and dual-head γ-cameras (4–6). All previous studies have used filtered backprojection (FBP) reconstruction for image processing. However, statistical image reconstruction algorithms, such as iterative methods, are becoming increasingly popular in the reconstruction of myocardial perfusion images. The most commonly used method is ordered-subset expectation maximization (OSEM), which is an ordered-subset implementation of the maximum-likelihood expectation maximization algorithm (7).
Iterative techniques have been shown to provide more accurate reconstructions than does FBP, even in cases of sparse or incomplete datasets (8,9). However, the effect of patient motion in the OSEM reconstruction method has not been studied. This study compared motion artifacts in the 2 reconstruction methods (FBP vs. OSEM).
MATERIALS AND METHODS
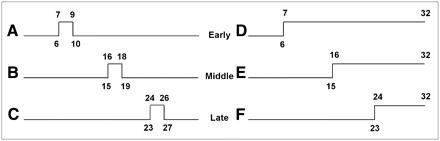
Six normal, motion-free 99mTc-methoxyisobutylisonitrile myocardial perfusion SPECT studies were selected according to the review of 3 nuclear medicine specialists. Absence of motion was documented by visual inspection of a rotating cinematographic display of the raw projections and summed images (linogram and sinogram). Scanning was performed on a single-head γ-camera (DSX; Sopha Medical Vision) equipped with a low-energy parallel-hole high-resolution collimator. The images were acquired in 32 projections with a 180° circular orbit from 45° right anterior oblique to 45° left posterior oblique, using step-and-shoot technique and 30 s of imaging per frame in nongated mode. The matrix size was 64 × 64, and the zoom factor was 1.33 (pixel size, 6.4 mm). No scatter or attenuation correction was applied to image reconstruction. Simulated patient motion was applied to all raw data with a displacement of 3 pixels in the x- and y-axes. The image set was displaced once in the x-axis and again in the y-axis, in a positive direction and in 2 types of shifting: returning and nonreturning (Fig. 1). The returning shift was applied for 3 consecutive frames only, resulting in 90 s of displacement. All simulated motion was applied in the early (frame 7), middle (frame 16), and late (frame 24) phases of acquisition. Accordingly, for the returning type of shifting, the early shift was in frames 7−9, the middle shift was in frames 16−18, and the late shift was in frames 24−26. For the nonreturning type, early, middle, and late shifting were from frames 7−32, 16−32, and 24−32, respectively. All original images and shifted images were saved as separate files and reconstructed using the FBP and OSEM methods. We used the Metz filter with a cutoff frequency of 4.8 (in full width at half maximum) and order 8 for all images. For OSEM reconstruction, 8 iterations and 2 subsets were used.
Schematic display of different kinds of motion used for simulation: early-phase returning shift (A), middle-phase returning shift (B), late-phase returning shift (C), early-phase nonreturning shift (D), middle-phase nonreturning shift (E), and late-phase nonreturning shift (F).
The reconstructed images were reviewed by 2 nuclear medicine specialists for the presence and locations of defects or the absence of defects. Imaging findings were categorized as normal, abnormal, or equivocal. The physicians were not aware of the reconstruction method used for each image. In cases of disagreement, consensus was reached with the review of the images by a third nuclear medicine specialist. Semiquantitative analysis was done for all images using a 17-segment model and a 5-point scoring system. However, basal segments were excluded, and 11 segments were considered in summation scores to exclude membranous septum variance.
Data from the 2 reconstruction methods were compared using the paired t test. The McNemar test and Fisher exact test were used for comparison of proportional data. Independent groups were compared using an independent t test or 1-way ANOVA. The Duncan test was used for multiple comparisons. A P value of less than 0.05 was considered statistically significant.
RESULTS
Seventy-two sets of shifted images were created: 36 sets for each axis, including 18 images with a returning shift (6 in the early phase, 6 in the middle phase, and 6 in the late phase of acquisition) and 18 images with a nonreturning shift. All images were reconstructed by the FBP and OSEM methods, yielding 144 images for interpretation.
The findings on the image sets were reported as either abnormal or equivocal using visual interpretation. With FBP reconstruction, 68.1% (49/72) of findings were considered abnormal, whereas 70.8% (51/72) of findings were abnormal using OSEM reconstruction.
Of the images with a returning shift, 44.4% (16/36) were reported as showing abnormal findings and 55.6% (20/36) as showing equivocal findings using FBP reconstruction. The percentage was 50% (18/36) in each group using OSEM reconstruction. Of the images with a nonreturning shift, 91.7% (33/36) were considered to show abnormal findings with both types of reconstructions (Table 1).
Qualitative Scan Interpretation
The paired t test was used to compare the mean summed score in the different types of reconstructions. In all shifted images, the mean summed score was 7.6 (±5.5) with FBP reconstruction and 8.5 (±5.5) with OSEM reconstruction (P < 0.001).
The mean summed score was lower with FBP reconstruction than with OSEM reconstruction in images with a returning shift (P < 0.001) but was not statistically different from OSEM reconstruction in images with a nonreturning shift (P = 0.13) (Fig. 2).
Mean summed score in returning and nonreturning shifts by 2 different reconstruction techniques. Statistical comparison was done between reconstruction techniques in each group.
The proportion of defects in each segment was compared for the 2 types of reconstructions using the McNemar test. The reconstruction technique had no significant impact on the location of defects (Table 2).
Number of Defects in 72 Shifted Images
The Student t test was used to compare the mean summed score between x-axis and y-axis shifting. The mean summed score was 7.93 (±4.7) for x-axis shifting and 7.29 (±6.2) for y-axis shifting (P = 0.629) using FBP reconstruction. The numbers were 8.66 (±5.1) and 8.48 (±5.9) using OSEM reconstruction (P = 0.891). The values for OSEM reconstruction were significantly higher than the values for FBP reconstruction in both the x-axis (P = 0.04) and the y-axis (P < 0.001). However, the location of the defects was not similar in x-axis and y-axis shifting (Table 3). With x-axis shifting, apicoseptal defects were seen more frequently, whereas with y-axis shifting, apicolateral and midinferior defects were more commonly seen.
Percentage of Defects in Each Segment
Figure 3 shows the severity and extent of defects (depicted as mean summed score) in relation to the frame of shifting. With a returning shift, the mean summed score was lower for early-phase shifting (frames 7−9) than for middle-phase shifting (frames 16−18) with both reconstruction types (P < 0.05). Similarly, the mean summed score was lower for late-phase shifting than for middle-phase shifting with FBP reconstruction (P = 0.02), but differences in mean summed score between these phases did not reach statistical significance with OSEM reconstruction (P = 0.07). Also there was no significant difference in mean summed score between early and late shifting with either reconstruction method (P > 0.22).
Mean summed score in shifted images compared in early, middle, and late phases of acquisition with respect to reconstruction technique and type of shifting. R = returning shift; NR = nonreturning shift.
With a nonreturning shift, the mean summed score was greater for shifting on middle- or late-phase frames than on early frames (Fig. 3), with both OSEM reconstruction and FBP reconstruction (P < 0.001). Also, the mean summed score was greater for middle-phase shifting than for late-phase shifting with FBP reconstruction (P = 0.004), but differences in mean summed score between these phases did not reach statistical significance with OSEM reconstruction (P = 0.39).
DISCUSSION
Patient motion commonly degrades SPECT myocardial imaging. Because of arthritis, weakness, and postexercise fatigue, patients usually have difficulty in hyperextending their left arm and remaining still for about 20 min, especially when imaged by a single-head γ-camera (10). Although previous studies noted that the tolerance of tomographic imaging to patient motion may be dependent on image acquisition and processing methods, only FBP reconstruction was used in those studies (4–6,10,11).
We studied normal, motion-free images and evaluated the pattern and severity of motion artifacts using the FBP and OSEM reconstruction methods.
We simulated patient motion by shifting planar images by at least 3 pixels on 3 consecutive frames. Our pilot study (12) and other studies showed that returning movements of fewer than 2 pixels may not produce significant perfusion defects (4,6,10,13). We also used at least 3 frames of shifting in the returning type of shift because it has been shown that even 20 pixels of movement on only 1 frame may not result in any defect (5).
This study showed that the number of images with definitely abnormal findings was slightly greater with OSEM reconstruction than with FBP reconstruction although not statistically significant (P > 0.5).
In addition, the summed mean score was larger with OSEM reconstruction than with FBP reconstruction, but the location of defects was not significantly different between the 2 reconstruction techniques. This finding suggests larger or more severe defects on images with OSEM reconstruction than on images with FBP reconstruction.
Matsumoto et al. (6) showed that a nonreturning shift produced more defects than did a returning shift. Similarly, our study confirmed their findings (Fig. 2) and showed that defects are more severe with OSEM reconstruction for a returning shift. For a nonreturning shift, the mean summed score was slightly higher with OSEM reconstruction than with FBP reconstruction but did not reach statistical significance. This finding suggests that although the difference between these 2 reconstruction methods is significant for mild defects, the difference is not significant for severe defects, which are seen easily with both methods.
Like previous studies (5,10), our study showed that the location of defects may differ according to the axis of motion (Table 3), but the severity and extent of defects were similar in both axes.
Although some researchers (6) have found that, with single-head γ-cameras, movement in the early phase of a study produces a greater number of false-positive defects than does movement in the middle or late phases, Cooper et al. (10) found that middle-phase movement was more significant than movement in the early or late phases. Our study showed that, with either type of reconstruction, movement in the middle phase of imaging produced more artifacts than did movement in the early phase of imaging. This finding is not surprising because when motion occurs toward the middle of the study, the images become more evenly split between projections of 2 different distributions of radioactivity, one before the motion and one after the motion.
In a recent work, Hatton et al. (9) showed that in cases of missing data (using a dual-head γ-camera), OSEM reconstruction demonstrates less severe artifacts than does FBP reconstruction and can tolerate at least 4 missing projections with no apparent effect on clinical interpretation. However, our study showed that motion artifacts are similar in location with both reconstruction types and that the extent and severity of artifacts (as depicted by summed scores) are significantly larger in OSEM reconstruction. Because missing data are fundamentally different from motion, which can be considered misregistered data, we do not expect to find similar results.
CONCLUSION
Motion artifacts do not differ in location between the 2 types of reconstructions (OSEM vs. FBP), but summed score is larger in OSEM reconstruction than in FBP reconstruction, suggesting larger or more severe defects using OSEM technique. OSEM reconstruction is not more tolerant than FBP reconstruction to motion artifacts.
Acknowledgments
We thank the research vice chancellor of the Mashad University of Medical Sciences for financial support of this research. We appreciate Hadi Jabbari's assistance in preparing the manuscript.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- Received for publication January 2, 2006.
- Accepted for publication May 15, 2006.