Abstract
Objective: The purpose of this study was to compare the accuracy and reliability of 2 well counter methods for measuring the activity concentration of 18F-FDG in blood samples.
Methods: Three to 5 blood samples from 154 patient studies were weighed and measured in a well counter. The 18F-FDG activity concentration was derived using, first, a direct calibration factor to convert measured well counter readings into activity concentration and, second, a comparison of measured counts with those of a specified standard solution.
Results: The ratio between the activity concentration results of the 2 methods was 0.996 ± 0.033, indicating that the methods provided equal results.
Conclusion: Because the standard solution method is more prone to human error, less reproducible, and more labor intensive, preference should be given to the direct calibration method.
Pharmacokinetic analysis of PET data requires exact determination of radioactivity concentrations in blood and plasma (1). Apart from possible online detection of continuously withdrawn blood (2), discrete blood samples are taken from the patient at fixed time points during the PET study. The activity concentrations of these samples are derived from measurements using a well counter. Two methods can be used to convert well counter readings into activity concentration: the standard solution method and the use of predetermined calibration factors.
The standard solution method is based on a solution of known radioactivity concentration, measured using a dose calibrator with a high accuracy (within 3%) (3). The activity concentration in blood samples is derived by simultaneously measuring standard solution and blood samples. The ratio between these measurements is used to derive the activity concentration in blood. This procedure is the accepted gold standard (4). The main advantage of this method is that it is independent of the performance of the well counter at the time of measurement. For example, any drift of well counter performance will automatically be considered. Use of this gold standard to determine activity concentrations in blood for PET studies is difficult, because most PET isotopes have a short half-life and because the method is labor intensive.
Independently measured calibration factors between well counter and PET scanner can also be used for determining activity concentrations. In this case, the counts measured with the well counter are simply multiplied by the calibration factor to obtain the activity concentration in the measured samples. The calibration factor method is simple and less labor intensive but depends on reproducible well counter performance. Therefore, well counters should be calibrated regularly. In our institute, the performance of well counters is checked daily using a 137Cs point source of about 7 kBq, and a cross calibration procedure, as described by Van Balen et al. (3), is performed monthly.
The purpose of the present study was to evaluate the usefulness of both methods for routine analysis of blood samples obtained during PET studies.
MATERIALS AND METHODS
Equipment
A VDC202 dose calibrator (Veenstra Instrumenten BV) was used for measuring activity in syringes. The well counters that were used included a Wallac 1470 Wizard (Perkin Elmer Lifescience) multiwell counter, equipped with 5 separate wells; a Wallac 1282 Compugamma (Perkin Elmer Lifescience) single-well counter; and a Wallac1480 Wizard (Perkin Elmer Lifescience) single-well counter, which was used after replacement of the Compugamma well counter. An XL410D balance (Denver Instrument Co.) was used for weighing the mass of the syringes. Blood and standard samples were weighed using a PC440 balance (Mettler-Toledo).
Cross Calibration Factors (CFs)
A cross calibration procedure was implemented to determine CFs between the ECAT EXACT HR+ PET scanner (CTI/Siemens) and the various well counters (3). The procedure was repeated every 2 wk, allowing assessment of relative reproducibility and accuracy of PET scanner and well counter performance. It was decided that the calibration factor would be adjusted when differences larger than 2% over a 3-mo period were found. Within this study, no such adjustments were required.
To derive CFs, a 5-mL syringe was filled with 100 ± 10 MBq of 18F-FDG. The exact amount of activity was measured in a previously calibrated dose calibrator. To avoid geometric effects from the dose calibrator measurements, the total volume of 18F-FDG in the syringe was always exactly 5 mL. The 18F-FDG was then injected into a water-filled calibration phantom with a volume of 6,283 mL, thus resulting in an activity concentration of ∼0.016 MBq/mL. Three samples, each of 0.5 mL, were then taken from this phantom. The weight of the samples was determined using a calibrated balance. Next, these samples were measured with each well counter. Combining the weight of the samples and the number of counts per minute resulted in the well counter rate concentration, Rwc (cpm/g), of these samples as determined by each well counter. Simultaneously, 2-dimensional and 3-dimensional emission scans of the calibration phantom were acquired with the PET scanner. After activity had decayed to background levels, a transmission scan was performed for the purpose of attenuation correction. Attenuation-corrected 2-dimensional and 3-dimensional emission scans were reconstructed using filtered backprojection and a Hanning 0.5 filter. Region-of-interest analysis was performed to derive the activity concentration in the phantom as measured by the PET scanner, ACPET (Bq/mL). Finally, the CFs between well counters and the PET scanner were calculated as follows:
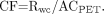 Eq. 1
Eq. 1
Note that the units of CF are (cpm/g)/(Bq/mL). Because 1 mL of water corresponds to 1 g, the unit for CF becomes cpm/Bq.
Within this study, the following CFs were used, as provided by Van Balen et al. (3): 27.2% ± 2.4% cpm/Bq for the Wallac 1470 Wizard, 20.2% ± 2.9% cpm/Bq for the Wallac 1282 Compugamma, and 47.4% ± 2.6% cpm/Bq for the Wallac 1480 Wizard.
Standard Solutions and Patient Dose Preparation
Standard solutions were prepared by first withdrawing about 0.1 mL from the daily 18F-FDG batch into a syringe. Exact weight and activity of the 18F-FDG in this syringe were measured. Next, this reference sample was diluted to a weighted total volume of about 35 mL. A second 1:8 dilution step was performed only for studies in which the volume of administered 18F-FDG was less than 2 mL, to obtain an approximate match between concentrations in standard solution and blood samples.
The patient doses were drawn into 5-mL syringes. Weight and activity were measured before dilution with saline to a volume of 5 mL. The latter step was performed to guarantee reproducible dose administration.
Corrections for background and leftovers were applied to dose calibrator measurements for both patient doses and standard solution syringes. Measurement times for both standard solution sample and patient doses were recorded.
Preparation of Well Counter Samples
A total of 6 weighted samples of 100 μL of the standard solutions, subsequently diluted to 550 μL, were prepared for measurement within the well counters. Weighed samples of 500 μL of both whole blood and plasma were prepared in duplicate. A volume of 50 μL 10% Triton-X (ICN Biomedicals Inc.) was added to the samples to destroy blood cells and obtain homogeneous solutions for measurement and avoid geometric effects caused by the formation of blood cell pellets.
Determination of Blood Sample Concentration
Using the standard solution method, activity concentration in blood and plasma samples was calculated according to:
 Eq. 2 where cpmsample is the cpm in the sample measured; msample is the mass of the sample measured, in grams; Vcount is the weight (volume) of the standard solution sample, in grams; Vtot is the total weight (volume) of the stock standard solution, in grams; Mstd is the mass of 18F-FDG in the stock standard solution, in grams; Mpat is the mass of 18F-FDG administered to the patient, in grams; cpmstd is the measured activity of the standard solution sample, in counts per minute; Dpat is the dose of 18F-FDG administered to the patient, in megabecquerels; and ACsample is the activity concentration of the patient sample, in megabecquerels per gram.
Eq. 2 where cpmsample is the cpm in the sample measured; msample is the mass of the sample measured, in grams; Vcount is the weight (volume) of the standard solution sample, in grams; Vtot is the total weight (volume) of the stock standard solution, in grams; Mstd is the mass of 18F-FDG in the stock standard solution, in grams; Mpat is the mass of 18F-FDG administered to the patient, in grams; cpmstd is the measured activity of the standard solution sample, in counts per minute; Dpat is the dose of 18F-FDG administered to the patient, in megabecquerels; and ACsample is the activity concentration of the patient sample, in megabecquerels per gram.
The density of the standard solution is assumed to be 1 g/mL. This explains the use of measured weights for the standard solution volumes. In Equation 2, it is also assumed that all activity measurements are decay corrected to the time of injection.
The results based on the CF were obtained by converting the sample activities (cpmsample) obtained by well counter measurements into a well counter rate concentration (cpmsample/msample, in grams) using the measured sample weights (msample, in grams). Again, a correction for decay between the time that the scan was acquired and the time that the blood sample was counted was applied. Finally, the activity concentration in the blood samples was obtained by dividing the obtained value by the CF of the well counter used:
 Eq. 3 where CF is the cross calibration factor of the used well counter (cpm/Bq).
Eq. 3 where CF is the cross calibration factor of the used well counter (cpm/Bq).
Comparison of Methods
Dynamic 18F-FDG studies were performed to obtain results for both calculation methods. For each study, the ratio between the activity concentration calculated using the standard solution method and the value according to the CF method (ACstandard/ACcross calibration) was calculated. The ratios were plotted versus several parameters, including date of acquisition and mass of patient dose, to search for possible trends. In addition, average ratio and SD were calculated for all studies.
RESULTS
Of 164 dynamic 18F-FDG studies, 155 were suitable for calculating activity concentrations using both calibration factors and the standard solution method. Nine studies were excluded because of errors made during the study, resulting in no or incorrect results for the standard solution method. For each study, 3–5 blood samples were taken. In a second screening of results, 8 studies with differences larger than 10% between blood sample concentrations calculated with the 2 methods were detected. In a third evaluation, 7 of those studies could be corrected, as differences were due to incorrect data entry. Only 1 study had to be excluded because of unexplainable results. Thus, 154 studies, providing 1,101 different blood and plasma samples, were used for evaluation. The ratio ACstandard/ACcross calibration was equal for each sample within a single study. When all errors were resolved, the average ratio between the concentrations calculated with the 2 methods for all studies (n = 154) was 0.996 ± 0.033, as shown in Figure 1. On 55 occasions, the ratio was larger than 1; thus, 99 times the ratio was smaller than 1, indicating a tendency for the CF calculated results to be slightly larger.
Ratio of activity concentration (AC) determined with standard solution and calibration factor method for 1 y. Slightly declining slope indicates that adjustment of calibration factors is required about yearly.
In Figure 2, the ratio of sample concentrations derived from the 2 methods is given as a function of the mass of the patient dose.
Ratio of activity concentration (AC) standard/calibration factor methods versus mass of patient dose. Observed large variation in this ratio is explained by uncertainties of measurements, which were required for preparation of standard solutions.
DISCUSSION
The evaluation of the standard solution and cross calibration methods showed that 10 of 164 studies needed further examination. In addition, the type of errors in the 7 corrected studies clearly indicated that the standard method and its more complex activity concentration calculation were more prone to human errors. Furthermore, the results in Figure 1 show a small increasing difference between the concentrations derived from the 2 methods over time. A small drift of the well counter or PET scanner performance could explain the latter observation. To overcome inaccuracy of the calibration factor method due to this drift, calibration factors are now validated about monthly (3).
Figure 2 shows that, for small masses, the variation of the ratio between the calculated activity concentrations using the 2 methods increases. This variation could be attributed to increased uncertainty of the standard method, primarily because of increased uncertainty in measured weights. Both weights of patient doses and reference samples are used in Equation 2.
CONCLUSION
Use of standard solutions with 18F is feasible, and the methods provide similar results. Because the standard method is more labor intensive and more prone to human error, calibration factors are preferred for routine use. The standard solution method, however, should be used if there are doubts about the stability of the well counter used.
Footnotes
For correspondence or reprints contact: Henri N.J.M. Greuter, PET Center, VU University Medical Center, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands.
E-mail: HNJM.Greuter{at}VUmc.NL









