Abstract
Objective: Although the use of 18F-fluorodeoxyglucose (FDG) PET for evaluating lymphoma is gaining in popularity, PET is not yet universally available and large prospective comparisons between 67Ga and 18F-FDG PET scans in predicting the long-term outcome after treatment are lacking. Scintigraphic imaging with 67Ga remains an important tool in evaluating the response of lymphoma to therapy. There are a variety of challenges and pitfalls inherent in 67Ga imaging for lymphoma. These are discussed and problem cases are illustrated. After reading this article, the nuclear medicine professional should be able to: (a) optimize the technical approach to and (b) maximize the diagnostic accuracy of 67Ga scintigraphy in assessing the response of lymphoma to therapy.
Gallium is a Group IIIb transition metal. Gallium-67 is widely used in nuclear medicine as a tumor-imaging agent by gamma-emission scintigraphy. Nearly 30 y ago, Edwards and Hayes (1) first described the accumulation of 67Ga in the lymph nodes of a patient with Hodgkin's disease. Subsequently, 67Ga has been shown to localize in a variety of tumors, as well as regions of inflammation, infection, and many skeletal disorders (2–13). In osteosarcoma, 67Ga may be useful for detecting soft tissue/lymph node metastases. Gallium-67 scintigraphy also has been applied to the diagnosis of pediatric soft-tissue tumors, melanomas, testicular tumors, and a variety of sarcomas (14). As a tumor-imaging agent, the use of 67Ga is best established in the diagnosis of lymphoma and hepatoma, where the overall sensitivity of a gallium scan is approximately 80% (15–17). Over the years, the role of 67Ga in nuclear medicine imaging for oncology has been almost exclusively focused on assessing the response of lymphomas to therapeutic maneuvers. This article addresses this specific application of 67Ga imaging and on strategies for improving the diagnostic accuracy of 67Ga scintigraphy in evaluating lymphoma.
WHICH IS BETTER: FLUORINE-18-FDG PET OR GALLIUM-67?
The Health Care Financing Administration (HCFA) has approved reimbursement for 18F-FDG PET for evaluating lymphoma. However, reimbursement is indicated in lieu of a 67Ga scan (either 18F-FDG PET or 67Ga will be reimbursed by Medicare and many third-party providers, but not both modalities for the same patient). Providers must choose between the two. With the increasing availability of PET nationally, many practitioners are turning from 67Ga to 18F-FDG PET for evaluating lymphoma, with the opinion that interpretation of PET is more rapid and timely (1 d as opposed to 3–7 d) and that 18F-FDG PET scans are easier to read than 67Ga scans. We agree with these opinions. However, one important fact must be emphasized. Although 67Ga certainly has limitations in evaluating lymphoma, years of good, reproducible research has been done to allow us to know exactly what those limitations are. With 18F-FDG PET, long-term prospective trials to assess the predictive value of a negative or positive scan after therapy, the optimal duration necessary to wait after completing therapy before scanning, whether the efficacy of therapy can be predicted by a scan performed early in the course of therapy, and the degree to which 18F-FDG PET contributes to the initial staging or the restaging of lymphoma are all questions for which there are, as of yet, no definitive answers. Early published reports and anecdotal experiences of many nuclear medicine practitioners with PET for lymphoma are promising, as PET is becoming more widely available (18,19). Large, reproducible, prospective, controlled trials are required before the ultimate utility of 18F-FDG PET can be compared with 67Ga, in staging and evaluating the therapeutic response of lymphomas of various types. Until the predictive value of positive and negative PET scans for lymphoma is clearly established, and until PET is available at all centers, many practitioners will still rely on 67Ga in evaluating lymphoma. A thorough understanding of the potential pitfalls of gallium imaging, the “tricks of the trade,” will add value to diagnosing and treating lymphoma by current methods.
APPROPRIATE PATIENT SELECTION
Appropriate patient selection is an important first step in maximizing the success of 67Ga scanning for lymphoma. The use of 67Ga is appropriate in all cases of Hodgkin's disease and for higher grade non-Hodgkin's lymphoma. The overall avidity of 67Ga in these patients is high. The magnitude of 67Ga uptake is highest in the nodular sclerosing form of Hodgkin's disease and in higher grade non-Hodgkin's lymphomas. Gallium-67 can be variable in its uptake, even between different individuals with the same histologic type of lymphoma. For this reason, it is important that a 67Ga scan be performed before therapy, to establish the gallium avidity of the tumor. Because 67Ga uptake by the tumor can decrease with even a single dose of chemotherapy, it is critical that a baseline gallium scan be performed before beginning therapy.
For low-grade non-Hodgkin's lymphoma, 67Ga uptake (as well as that of 18F-FDG) is variable. Although many advocate the use of 67Ga for low-grade lymphomas (20,21), many practitioners prefer to use 201Tl or 99mTc-sestamibi for patients with lymphoma, particularly low-grade lymphoma (22,23). In 30% of patients who initially have low-grade lymphomas, conversion to a higher grade lymphoma occurs and is often associated with the development of strongly gallium-avid tumor (24). For non-Hodgkin's disease of intermediate grade, a 67Ga scan performed before any therapy should establish whether the tumor is gallium avid. If it is, then 67Ga likely can be used to assess the response of this specific tumor to future therapy.
The success of the 67Ga scan will depend on understanding the specific clinical question being asked before undertaking the imaging study. There are 3 general categories of questions asked of the nuclear medicine practitioner for patients with a new diagnosis of lymphoma. First, what is the initial stage of the lymphoma? Second, is the chosen therapeutic approach appropriate for the patient? In other words, can the response of the tumor be predicted early in the course of treatment? Third, has the tumor been completely eradicated by the therapy used? For patients who have been successfully treated previously and who have recurrent symptoms or new radiographic findings, 3 questions also are asked frequently: has the tumor recurred; if so, what is its stage; and has it transformed into a higher grade?
What Is the Stage of the Lymphoma?
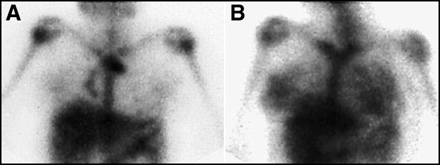
For the initial staging of lymphoma, it is well accepted that 67Ga imaging is considerably inferior to anatomic imaging, such as conventional CT (25). An initial 67Ga scan, in patients with newly diagnosed lymphoma, should be done to establish the gallium avidity of the tumor, not to stage the patient. In patients who have previously been treated for lymphoma and have suspected recurrence, the use of 67Ga for staging is much more useful. Radiographic findings are likely to be abnormal as a result of the previously treated tumor, with fibrotic masses at sites of previous bulky disease, postsurgical and radiation changes, and scarring (26). However, even with initial staging, the information gained by the 67Ga scan occasionally can change the stage of the tumor, as illustrated in Figure 1. For this reason, it is important that the nuclear medicine practitioner have a clear understanding of lymphoma staging systems. There are several systems for the staging of lymphoma, both for Hodgkin's and non-Hodgkin's, and the practitioner should be familiar with all of them. Table 1 illustrates one of the most widely used classifications for Hodgkin's disease, the Ann Arbor Classification. With the increasing stage of the disease, the prognosis worsens and the therapy may change. The presence of B symptoms of weight loss, fever, and night sweats also worsens prognosis.
The 67Ga scan can be useful as an adjunct to staging. Two hilar nodal groups were suspected in this patient with Hodgkin's disease, who would have been assigned a stage II classification. Gallium-67 images of the chest, (A) anterior planar and (B) posterior planar, also reveal additional nodal groups and the presence of multiple bony mets, placing this patient in a stage IV category.
Hodgkin's Lymphoma Staging: Ann Arbor Classification
Can the Tumor's Response Be Predicted Early in the Course of Treatment?
There are several studies that demonstrate the efficacy of a 67Ga scan in predicting the response of the lymphoma to treatment, either early or in midcycle of treatment (27–30). This approach is most beneficial in a specific subset of patients: those with a poor prognosis or aggressive disease who have a persistent or worsening signs or symptoms despite the recent initiation of therapy. In these patients, it would be useful to know as early as possible if the therapy chosen was bound for failure, so that changes could be made to maximize the patient's chance of survival.
It is not entirely clear how early in the course of treatment a 67Ga scan can be performed that will predict a tumor's response. Advocates of this approach claim that imaging can be done after 2–4 cycles, or midway in a round of chemotherapy. There are actually reports of patients who had been injected with 67Ga, had early imaging that demonstrated gallium-avid tumor, then had a single dose of chemotherapy, with subsequent images being remarkable for a disappearance of 67Ga from the tumor. The mechanism for an early decrease in 67Ga uptake with appropriate treatment is not clear, because it is likely that the tumor is nonetheless still viable. It is possible that these changes occur as a result of internalization of transferrin receptors, which exist both on the surface of the cells and within a cytoplasmic pool. Rapid changes in externalization of the transferrin receptor, which may be responsible for uptake of 67Ga by tumor cells, may occur in response to cell cycle or proliferative activity, and not just because of metabolic activity or cellular viability.
Has the Tumor Been Completely Eradicated by the Therapy Used?
The most common, and best-supported use for 67Ga imaging is in predicting adequate sterilization (eradication) of the tumor after completion of radiation and chemotherapy (16,31). This use of 67Ga is illustrated in Figure 2. Because imaging can fail to reveal the presence of tumor after even a single dose of chemotherapy, it is important to wait a reasonable interval after completing therapy to allow any remaining viable tumor to regain metabolic or proliferative activity. It has been recommended that injection of 67Ga be delayed 3–6 wk after completion of chemotherapy or radiation (17). However, many clinicians are reluctant to wait this long, because the initiation of additional chemotherapy should be as timely as possible, if residual tumor is still present. Anecdotally, many nuclear medicine practitioners are willing to reduce the requisite interval to 2 wk after completion of therapy. This should be considered the absolute minimum. It must be reemphasized that injection of 67Ga at intervals less than 3 wk after completion of chemotherapy could lead to a false-negative exam, when the exam is performed to determine whether the tumor has been completely eradicated by the chosen therapy. Table 2 is an average taken from numerous reports, all of which are in good agreement, of the predictive value of survival (with or without disease) with a positive or negative 67Ga scan performed at the completion of therapy for lymphoma. Data such as these are needed for 18F-FDG PET.
Successful treatment of lymphoma is demonstrated by 67Ga scan. (A) This patient had bulky mediastinal and supraclavicular disease before therapy. (B) After therapy no residual disease is found.
Predictive Value of a Gallium-67 Scan After Therapy: Survival at 24 Mo Post-Therapy*
PATIENT PREPARATION
Beyond the appropriate timing of injection of 67Ga relative to the timing of chemotherapy, there is little patient preparation needed for a 67Ga scan. There are reports that suggest that the administration of iodinated contrast should not be performed on the same day as injection of 67Ga: the contrast agent may bind 67Ga and result in more rapid elimination of the tracer. Although colonic activity commonly complicates the interpretation of a 67Ga scan, the administration of cleansing bowel preparations or enemas does not improve this situation. In fact, harsh laxatives and enemas may cause irritation and further enhance uptake by the colon. It is better if the patient is simply instructed to maintain a good diet that is high in fiber and drink plenty of liquids. Delayed imaging is the only very reliable way to eliminate bowel activity. Care should be taken to avoid commencing a 67Ga scan in a patient with an intercurrent infection, recent line placement, surgery, or other causes of inflammation to avoid false-positive tumor scans caused by inflammation or infection.
TECHNICAL CONSIDERATIONS AND TIMING
Table 3 illustrates the appropriate doses of 67Ga citrate, and the typical timing of imaging for lymphoma patients. For infection or inflammation, imaging after injection of 67Ga can often be accomplished at 48 h, or even 24 h, after injection. For tumor imaging, the initial set of planar images should be delayed until 72 h postinjection. Planar imaging is performed in the anterior and posterior projections, to include the head, neck, chest, abdomen, pelvis, and proximal extremities. The distal extremities can be included as clinically indicated. Lateral views of the head and neck are also routinely acquired at 72 h and can include the ipsilateral axilla, with the arm abducted. SPECT imaging of the neck and chest should be performed at 72 h postinjection. We routinely use SPECT scanning with 67Ga. There is considerable evidence that SPECT scanning improves the sensitivity and specificity of 67Ga scanning both before and after treatment of lymphoma (32). If SPECT is not to be performed, the late William Kaplan, MD, of the Dana-Farber Cancer Institute, advocated an anterior, posterior, and, if necessary, lateral image of the chest for 1000 K counts at 72 h postinjection. These images should be performed in the upright position with the arms extended laterally on the anterior view, embracing the camera. This improves visualization of the thoracic structures. The upright position is important in evaluating for the presence of cardiophrenic nodes.
Typical Imaging Sequence
Most patients will have bowel activity at 72 h (3 d) after injection. This being the case, it is recommended that a second set of planar images of the abdomen be obtained at 5–7 d postinjection, to ensure adequate evaluation of the abdomen (33). SPECT imaging of the abdomen can be performed at that time, if gut activity has cleared. Because Medicare and many third-party payers do not reimburse for more than 1 67Ga SPECT exam per day, delaying SPECT imaging of the abdomen not only allows time for clearing of the bowel activity, it ensures that appropriate billing and reimbursement can occur. Although 67Ga activity can still be seen as long as 3 wk postinjection, it is unlikely that adequate activity will be present to allow satisfactory SPECT imaging at intervals longer than 5–7 d postinjection.
Because extended imaging is often required for 67Ga scans, the dose of radionuclide used is usually higher than is needed for 67Ga imaging performed for reasons of inflammation or infection. In the latter 2 instances, an adult dose of 5 mCi is typical. For imaging lymphoma, an adult dose of 8–10 mCi is more appropriate. For children, a weight-adjusted dose of 75 μCi/kg is appropriate, as shown in Table 3.
The exact parameters used for both planar and SPECT imaging of 67Ga will vary somewhat, depending on the specific make and type of camera used, the collimator specifications, and processing algorithms and display software available. These are often proprietary and may vary from vendor to vendor. In Tables 4–6, examples of the parameters used for the acquisition and processing of planar and SPECT images using a large field-of-view, dual-headed Picker SPECT camera are given. It must be stressed, however, that the nuclear medicine technologist should start with the manufacturer's recommendations and only then cautiously apply variations to optimize the images. As is universally true in nuclear medicine imaging, the principles that are most important are to place the camera as close to the patient as possible, acquire as many counts as are tolerable to the patient without movement or discomfort, and display both planar and SPECT images with intensities carefully chosen to place regions of interest in the linear portion of the curve relating activity to intensity.
Acquisition Parameters for Planar Images
SPECT Acquisition Parameters
SPECT Process/Display Parameters—SPECT*
NORMAL AND ABNORMAL PATTERNS OF UPTAKE
Head and Neck
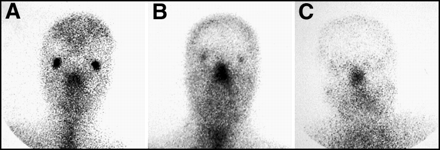
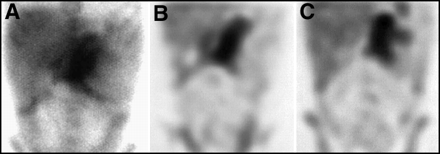
There are normal variations in the appearance of a 67Ga scan of the head. It is normal for patients to have diffuse nasopharyngeal activity. Stomatitis, related to chemotherapy or radiation, could cause more intense activity in the mouth. Focal intense activity in the posterior oropharynx should be regarded with suspicion for possible nodal disease in the tonsillar region. The appearance of the lacrimal glands on a 67Ga scan can be quite variable, as shown in Figure 3. Activity can be absent or intense. This is thought to be proportional to the degree of lacrimation between the time of injection to imaging, with greater activity seen in patients who have been crying, or who experience enhanced lacrimation from contact lenses or irritation. For patients in whom there is a question of retro-orbital lymphoma, a SPECT acquisition would be required to distinguish retro-bulbar from lacrimal gland activity.
Normal patterns of facial uptake of 67Ga are variable. Activity in the lacrimal glands can range from (A) intense to (B) moderate to (C) absent.
Figure 4 illustrates another pitfall in evaluating the head and neck of a patient with lymphoma. Sialadenitis is a common side-effect after chemotherapy or radiation. This can result in intense uptake in the submandibular and parotid salivary glands. This should not be confused with cervical adenopathy. A strategy for confirming salivary, as opposed to lymph node, activity at these sites would be to compare the 67Ga distribution with that of 99mTc-pertechnetate. The latter would be expected to accumulate in salivary glands, but not lymph nodes. The converse problem is illustrated in Figure 5: the presence of abnormal lymph nodes that could be confused with salivary glands. Submental or high cervical lymph nodes containing lymphoma could acculate 67Ga and be confused with sialadenitis. In addition, reactive inflammatory lymph nodes in the neck could accumulate 67Ga without containing lymphoma. Any unexpected activity in the neck should prompt further clinical inquiry to establish the correct etiology.
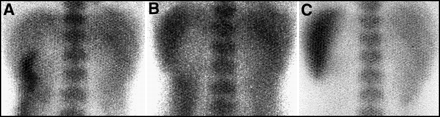
Sialadenitis is a common complication after chemotherapy and radiation. (A) Anterior and (B) lateral planar views of the head and neck before therapy demonstrate a large left neck tumor. After therapy, the tumor is eradicated but there is intense activity seen on both the (C) anterior and (D) lateral planar images in the parotid and submandibular glands. This is not lymph node activity.
Submental lymph nodes or high cervical lymph nodes containing lymphoma can often be mistaken for salivary gland activity. Shown here, in a patient with a superior mediastinal tumor, is lymphoma in submental lymph nodes seen on both the (A) anterior and (B) lateral planar images.
Heart
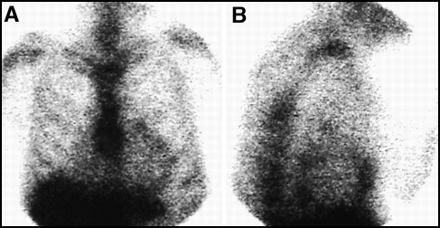
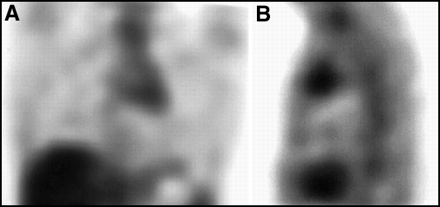
There should be no cardiac activity on a 67Ga scan performed ≥ 48 h postinjection. Any activity in the heart should be considered abnormal. Figure 6 illustrates an abnormal pattern of uptake circumferentially located around the heart. This pattern would suggest tumor involving the pericardium, although a similar pattern also could be seen with infection or other inflammation of the pericardium.
No cardiac activity should be seen on a 67Ga scan. Shown here, in both (A) anterior planar and (B) lateral planar views is activity around the heart, consistent with a malignant effusion of the pericardium.
Lung
A normal 67Ga scan should have no lung uptake, as shown in Figure 7A. Diffuse mild lung uptake, as shown in Figure 7B, however, is a common and usually normal finding in patients after chemotherapy (34). The duration of the lung uptake after chemotherapy is not clear, and it is our experience that the activity may persist for several months. The reason for the mild lung uptake is unclear. Conventional chest radiographs and high-resolution CT scans in these patients are usually normal or noncontributory. In immunocompromised patients, who have been treated with aggressive chemotherapy and possibly bone marrow transplantation, the possibility of opportunistic infection also should be considered. This is illustrated in Figure 7C, a 67Ga scan in a patient with Pneumocystis pneumonia, and in Figure 7D, after treatment.
(A) A normal 67Ga scan of the chest should reveal no lung uptake. (B) However, mild diffuse activity in the lungs is common and normal after chemotherapy. If activity is intense or the patient is immunocompromised, other inflammatory or infectious conditions should be considered. An example shown here is PCP pneumonia, shown (C) before treatment and (D) after treatment.
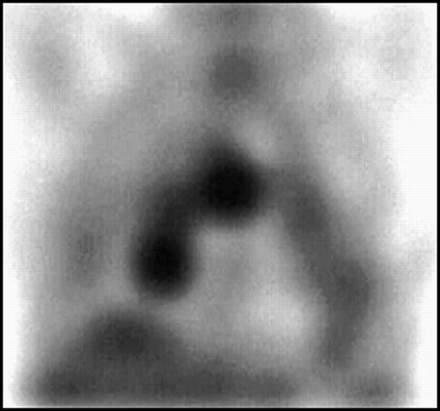
Patchy lung activity is not normal. It either represents tumor, inflammation, or infection. It is important to remember that 67Ga is a useful agent in imaging both infectious and noninfectious inflammatory conditions of the lung, as is illustrated in Figure 8, a patient with a lung abscess.
Focal lung uptake should always be regarded as abnormal, but can be from either tumor or infection. Shown here in (A) anterior and (B) lateral planar images is a left lower lobe lung abscess. Note the air:gallium level.
Pulmonary Hilar and Mediastinal Structures
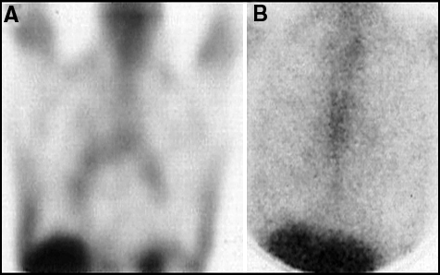
The mediastinum and hila of the lungs are common places for initial occurrence of lymphoma. Specific attention to these areas is critical on both initial and follow-up 67Ga scans. Asymmetrical or intense activity after therapy, as illustrated by the coronal SPECT image in Figure 9, is highly suspicious of the presence of lymphoma. However, it is very common for patients who have been treated with chemotherapy to have mild, diffuse bilateral hilar and mediastinal uptake, as is shown in the coronal SPECT image of Figure 10A. The intensity of uptake on SPECT images is sometimes difficult to judge because the automated intensity settings may artifactually increase the apparent degree of uptake. However, the planar view of the chest in this same patient (Figure 10B) shows the symmetrical hilar/mediastinal uptake to be very faint. Faint, symmetical hilar uptake on the 67Ga scan is a normal finding after chemotherapy and should not be confused with tumor (35,36). The initial 67Ga scan in smokers often reveals a similar pattern of symmetrical and mild uptake. This is likely related to nonspecific inflammatory changes from smoking. CT usually shows no suspicious lymph nodes in these patients.
Focal, asymmetrical or intense hilar uptake is consistent with lymphoma, as shown in this coronal SPECT view.
Normal mild symmetrical hilar uptake can be normal in smokers, or in many patients after chemotherapy and radiation, as shown here in (A) coronal SPECT and (B) planar views. The intensity of uptake on the SPECT views may overestimate the magnitude of uptake and reference to the planar view should always be made.
Although the traditional rule of Oslerian medicine is that patients have but 1 disease, it is nonetheless important to remember that significant uptake in lymph nodes also can be seen with a variety of diseases other than lymphoma. These include many other types of tumors, infections that result in lymphadenopathy, and both infectious and noninfectious granulomatous disease. An example of the latter, a patient with sarcoidosis, is shown in Figure 11.
Many diseases may mimic lymphoma, including other tumors and both infectious and noninfectious granulomatous disease. (A) Shown here is a chest radiograph of a patient with hilar and mediastinal fullness, (B) with uptake on 67Ga scan in the same regions. The diagnosis here was sarcoidosis.
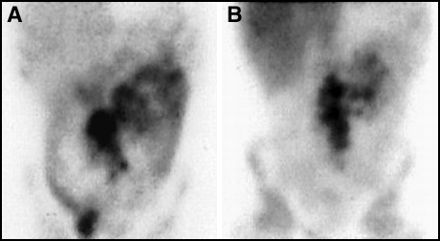
In children and young adults, the phenomenon of rebound thymic hyperplasia has been well described after chemotherapy (37). This condition usually is seen in patients who are treated with chemotherapy, but not radiotherapy, to the mediastinum. It usually is seen in children but has been described in patients as old as their early 20s. Thymic rebound can result in uptake of 67Ga in the thymus in a pattern that can be confused with residual or recurrent lymphoma to the mediastinum. Such a case is illustrated in Figure 12. It has been suggested that a follow-up scan in 2–3 mo will demonstrate regression of activity if the abnormality is because of thymic rebound, and not lymphoma.
Rebound thymic hyperplasia can occur after chemotherapy in children and young adults and can result in uptake on 67Ga scan in the anterior, superior mediastinum, as shown here in both (A) coronal and (B) sagittal SPECT views. A follow-up scan in 2–3 mo will show regression of activity in “thymic rebound.”
Breast
It is normal for breast tissue in developing or mature women to accumulate a small amount of 67Ga. We and several of our colleagues have anecdotally observed that relatively intense uptake is often seen in the breasts of developing girls on the postchemotherapy scan. One such example is illustrated in Figure 13. A possible explanation for this finding is a hormonal rebound, which may occur after chemotherapy. We have not observed this same finding in sexually mature women. Although it is rare, primary and metastatic lymphoma to the breast has been described (38,39). Therefore, an adequate physical examination for clinical correlation is appropriate whenever uptake by the breast is observed. Obviously, focal or unilateral uptake would be regarded with greater suspicion for either a malignant or inflammatory etiology than would symmetrical uptake.
Mild breast activity can be normal on 67Gan scintigraphy. However, intense breast activity can occur in developing girls after chemotherapy, possibly from hormonal rebound. Shown here is a girl with mediastinal lymphoma (A) before and (B) after chemotherapy. Also note the suppression of activity in the epiphyseal plates after chemotherapy. This is also a common finding.
Axilla
When imaging the chest, it is necessary to obtain an image in the anterior projection with the arms abducted and elevated. With the arms down at the side, additive activity of the axillary skin folds can mimic axillary adenopathy, as illustrated in Figure 14. This same phenomenon also has been observed with 18F-FDG PET scans. With peripheral adenopathy, it is vitally important that a good physical examination be performed. Despite our best efforts, the sensitivity of 67Ga for detecting abnormal peripheral lymph nodes that are within palpable regions is less than that of the physical exam.
Skin folds in the axilla can mimic abnormal lymph nodes. An anterior view of the chest with the arm extended will abolish the finding if activity is due to superimposed tissue from axillary skin folds, shown here for both the (A) right and (B) left axilla.
Distinguishing Bowel from Mesenteric Lymph Nodes
Gallium-67 is excreted by the upper large intestine. Therefore, bowel activity on a 67Ga scan should be confined to the colon, and not appear within the small bowel or mesenteric distribution. However, the colon on many patients can be somewhat redundant and can significantly compromise detection of tumor within the abdomen (Fig. 15A). The best solution for circumventing this problem is to perform delayed imaging, as illustrated in Figure 15B–C, and SPECT. As noted above, delayed imaging performed beyond 5–7 d postinjection is unlikely to be productive of sufficient count density to allow SPECT. It is better to perform a SPECT study of the abdomen within the first week postinjection, even if the bowel activity has not entirely cleared.
(A) Diffuse bowel activity can often compromise interpretation of the 67Ga scan. (B) The typical distribution of activity should be confined to the large intestine. (C) Delayed imaging at 5–7 d postinjection usually will result in clearing of most of the activity from the bowel.
Figures 16 and 17 demonstrate abnormal patterns of colonic activity that are suspicious for mesenteric tumor. These include central collections that are clearly not colonic in distribution, as shown in Figure 16. Round foci of activity may be indicative of scattered mesenteric nodes, as shown in Figure 17 from a patient who also had mediastinal and hepatic areas of lymphoma. Basically, anything not clearly within the colon should be regarded with suspicion. With respect to the abdomen in general, correlative imaging with CT is imperative in improving the sensitivity and specificity of the 67Ga scan of the abdomen.
(A) Activity that is centrally located, and not typical of the distribution of the colon, should be regarded as suspicious for lymphoma. (B) Delayed imaging should result in clearing of normal bowel activity, but not that in the tumor, as is shown in this patient with a large mesenteric nodal mass.
Lymphoma-containing nodes may stud the mesentery, producing focal areas of uptake that are atypical for the appearance of bowel, as is shown in planar views of the (A) upper and (B) lower abdomen in this patient who had multiple areas of tumor.
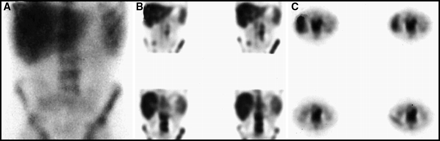
The detection of periaortic and aorto-caval lymphomatous lymph nodes is difficult because they lie on the anterior aspect of the vertebral body and can be confused with osteophytes, which also can show enhanced uptake on the 67Ga scan. SPECT imaging and, again, careful correlation with plain radiographs or CT are very important in maximizing the accuracy of detecting periaortic lymph nodes. An example of periaortic lymph nodes is illustrated in Figure 18.
Periaortic lymph nodes may be difficult to identify on planar views (A) because they are closely applied to the anterior aspect of the vertebral bodies. (B, C) SPECT imaging in multiple planes may help identify the nodes as being distinct from the vertebrae. It is important to correlate with radiographs to exclude the presence of large anterior osteophytes, which can mimic periaortic lymph nodes.
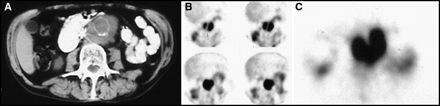
In patients who have abdominal aortic aneurysms or vascular grafts, a mass around the aorta also can be confused with a leaking aneurysm or infection of a vascular graft. Although patients with a suspected acutely leaking aneurysm would likely be evaluated by other more expeditious imaging methods, a 67Ga scan could distinguish between an old organized hematoma and a mass that is caused by lymphoma, as illustrated in Figure 19. An infected vascular graft would be expected to demonstrate uptake of 67Ga as well.
This patient had a vena caval filter and a history of an aortic graft as well as lymphoma. He was asymptomatic and hemodynamically stable. (A) CT scan showed a large periaortic mass, which was 67Ga-avid, as shown on SPECT (B) coronal and (C) transaxial images. The mass was recurrent lymphoma.
Kidneys
In healthy patients, renal activity can be considered normal up to 48 h after 67Ga injection. Delayed, symmetrical renal activity can be seen in patients with interstitial nephritis, nephrotic syndrome, or with other forms of renal insufficiency. Most patients who have been treated with chemotherapy also demonstrate mild symmetrical renal activity, as is shown in Figure 20A. In our experience, this finding persists indefinitely and is not abolished by delayed imaging. Suspicion for renal involvement with lymphoma should be raised when kidney activity is intense, asymmetrical, or associated with enlargement of the kidney (Fig. 20B). Care must be taken to ensure the patient does not have a pyelonephritis at the time of imaging, which can cause focal or diffuse uptake in a kidney, as shown in Figure 20C.
(A) Mild diffuse renal activity is normal after chemotherapy, as in this patient who also had a prior splenectomy. (B) However, if renal activity is intense or there is enlargement of the kidney, as in this coronal SPECT image, lymphoma should be suspected. (C) It is important to ensure that renal activity is not related to pyelonephritis, which can result in uptake on the 67Ga scan.
Liver and Spleen
The 67Ga scan is of very limited use in diagnosing lymphoma involving the liver and spleen. Lymphoma is often diffusely infiltrating in its nature in these organs. Unless focal collections of activity are seen, the diagnosis of lymphomatous involvement cannot be reliably made (40). On a conventional 99mTc sulfur colloid scan, the spleen should be similar to, or of less intensity than, the liver on the posterior image. This is also true of the spleen on the posterior image of a 67Ga scan at 3 d postinjection. If the spleen is hotter than the liver and is enlarged at 3 d postinjection, there should be a high suspicion for lymphoma involvement (Fig. 21). However, on images delayed >3 d, even a normal spleen increases in intensity relative to the liver, as shown in Figure 22. For this reason, delayed images beyond 72 h are inaccurate in predicting splenic involvement. As is true for 99mTc sulfur colloid, the spleen also can demonstrate increased uptake, relative to the liver, for a variety of causes, and the finding is not specific for lymphoma.
It is often difficult to determine if the spleen and liver are involved with lymphoma because of its typically diffusely infiltrating nature. If the spleen is “hotter” than the liver 3 d postinjection of 67Ga, as shown in this patient who also has mediastinal lymphoma, splenic involvement should be suspected. However, any cause of hypersplenism also can cause increased splenic uptake.
If imaging is extended beyond 3 d postinjection of 67Ga, the spleen normally becomes progressively “hotter” with time, relative to the liver, as shown here at (A) 3, (B) 5, and (C) 7 days postinjection. This is a normal finding.
Stomach
It is relatively common to see diffuse stomach uptake on a 67Ga scan in a patient who has received therapy for lymphoma, as is demonstrated in Figure 23. This has been described in the literature and is said to occur in nearly 10% of lymphoma patients examined post-therapy (41). Whether the patients should undergo endoscopy should depend entirely on their clinical symptoms. If activity is diffuse and they are asymptomatic, then no further work-up is needed. If they have gastric symptoms, then endoscopy should be performed. Symptomatic patients often are found to have pathology on endoscopy, either gastritis or tumor involvement. Focal, rather than diffuse, uptake in the stomach should be regarded with greater suspicioun and is more likely to represent lymphoma, as illustrated in Figures 24 and 25.
Diffuse gastric uptake can be normal after chemotherapy and can be seen in up to 10% of patients. Anterior planar views of the abdomen (A) before and (B) after administration of a carbonated beverage illustrate this finding. Endoscopy is indicated only if the patient is symptomatic.
Focal gastric uptake should be regarded as suspicious for lymphoma, as illustrated in this patient with Burkitt's lymphoma of the stomach. Shown here are (A) planar anterior and (B, C) SPECT coronal views of the abdomen with a large 67Ga-avid mass in the stomach region.
Focal gastric uptake involving cardia of the stomach in a patient with stage IV lymphoma. There is also extensive tumor involving the mediastinum, a right cardiophrenic node, and the kidneys.
Skeleton
Gallium-67 is a weak bone imaging agent. The normal distribution of 67Ga in the skeleton should be similar to that of a 99mTc-bisphosphonate bone scan, with activity in the mineral (cortical) portions of the bone, not the bone marrow. Any cause of increased uptake on a conventional bone scan also can cause abnormal accumulation on a 67Ga scan. For example, degenerative disease of the spine or joints can result in an abnormal pattern of 67Ga uptake, as is illustrated in Figure 26. Careful correlation with CT or plain films is essential in improving the specificity of 67Ga in diagnosing skeletal lymphoma.
Degenerative arthritis, both of the joints and spine, can result in enhanced uptake of 67Ga, as shown by (A) planar and (B) coronal SPECT imaging of this patient with arthritic changes of the hips and lumbar spine (shown by radiographs).
In skeletally immature individuals, it is normal for the epiphyseal plates of the bones to have greater uptake than other portions, as is also true for conventional bone scans. It has been our observation that after chemotherapy it is common for the magnitude of activity in the growth centers of the bones to be depressed on the 67Ga scan. An example of this finding is shown in Figure 13. The decrease in epiphyseal activity may be due to nonspecific suppression of all actively dividing cells, including those at the epiphyseal plates. With time, the activity usually returns to normal.
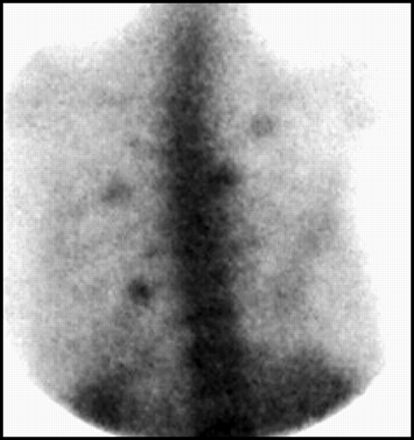
SPECT imaging of the spine, particularly the lumbosacral spine, is fraught with difficulties (42). Attenuation of activity due to the liver often produces a relatively photopenic segment of spine in the upper lumbar region, as shown in Figure 27A, with accentuation of activity in vertebral bodies immediately above or below the photopenic segment. Count-poor images often appear patchy, as illustrated in Figure 27B. Because of the great number of false-positives in the lumbosacral spine, it is our policy to require that an abnormality also be demonstrated on the planar images to be considered convincing, as is shown in Figure 28.
The lumbosacral spine can be difficult to evaluate by SPECT. Typical normal patterns may include (A) segmental attenuation of activity by the liver and (B) patchy uptake due to poor counting statistics or obesity.
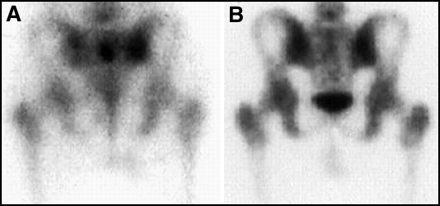
Focal abnormal uptake of the lumbosacral spine should be seen both on (A) planar and (B, C) SPECT views, as is demonstrated in this patient with lymphoma involving a midlumbar vertebra.
Gallium-67 also is actively accumulated in bones with acute or healing fractures or with infection. These can complicate a determination of the extent of lymphoma. This presents a dilemma for both radiologists and nuclear medicine physicians because CT and MRI will often be abnormal in these bones as well, and it may be difficult to identify a confirmatory noninvasive study that will confidently distinguish fractures from malignancy in all cases. Figure 29 illustrates a patient with suspected stage I lymphoma with uptake in several ribs due to recent fractures. Bone infections also can result in false-positive 67Ga scans for lymphoma. Figure 30 illustrates a patient with an osteomyelitis of the sacroiliac region, probably caused by a marrow biopsy performed several weeks earlier.
Focal rib uptake due to fractures, as seen in this patient, can mimic lymphoma involving the ribs.
Infection can mimic lymphoma on a 67Ga scan, as is shown in this patient with an osteomyelitis and abscess of the sacroiliac region, due to a prior bone marrow biopsy.
Focal uptake of 67Ga in a skeletal region that does not correspond to an obvious abnormality on a 99mTc bone scan or plain films, as shown in Figure 31, is not uncommon and is usually due to involvement of the marrow with tumor, but not the cortical portion of the bone. The 67Ga scan may be significantly more sensitive for detecting bone involvement than is the conventional bone scan (43,44). If confirmatory imaging is required to define an abnormality seen on the 67Ga scan, but not on plain film, an MRI usually will demonstrate abnormal marrow signal at this site if tumor is present. Many patients may have had a bone marrow biopsy or bone marrow harvest as part of their evaluation. These maneuvers can produce abnormal uptake at the site of the biopsy (as is also true after soft-tissue biopsy or line placement) (45). However, any asymmetry in skeletal activity should be investigated with correlative clinical information or imaging to exclude lymphomatous involvement.
Lymphoma of the bone often involves the marrow before extending to the bony cortex. (A) 67Ga scan is often more sensitive for detecting lymphoma of the bone than is (B) conventional 99mTc bone scan.
When the marrow is diffusely 67Ga-avid, this may represent diffuse lymphoma of the marrow, especially on a pretherapy scan. However, the finding of diffuse marrow activity is common on post-therapy scans in patients who have received, as part of their chemotherapy regimen, granulocyte colony stimulating factor (GCSF) or erythropoietin (46). Often, bone or bone marrow areas previously involved with lymphoma, previously irradiated, and successfully treated will appear as cold defects on these post-therapy scans, as illustrated in Figure 32. We have observed enhanced marrow activity on 67Ga scans 1–2 y after administration of GCSF or erythropoietin. It is not clear if, or when, the activity returns to normal. When marrow activity is visualized, a complete history is required to determine the significance of the activity.
Diffuse marrow uptake of 67Ga can be seen in patients with diffuse lymphoma of the bone marrow. However, diffuse marrow uptake is a common finding in patients who have previously received chemotherapy that included granulocyte colony stimulating factor (GCSF) or erythropoietin. An example of a patient treated with GCSF is shown here. (A) Planar thoracic, (B) pelvis, and (C) femur views of this patient demonstrate bony activity confined to regions typical of those for red marrow, as illustrated by (C) where activity extends only to the mid-femur. Areas of previous and successfully treated lymphoma involving the skeleton, or areas of prior radiation, will often appear as cold defects, as in (B), where the patient had been irradiated for a large left inguinal mass with associated bony involvement of the left ischium.
SUMMARY
Although 18F-FDG PET may replace 67Ga for evaluating lymphoma in the future, PET is not yet universally available. The predictive value of FDG in assessing the early and long-term response to therapy, as well as its value in staging (compared with conventional modalities) are areas in need of concentrated research. Gallium-67 remains an important modality in evaluating lymphoma, at least for the present time. Careful attention to appropriate patient selection, timing, and techniques of imaging; common patterns and pitfalls; and meticulous correlative imaging are important to maximize the sensitivity and specificity of 67Ga imaging for lymphoma.
Footnotes
For correspondence or reprints contact: Kathryn A. Morton, MD, Department of Radiology, Division of Nuclear Medicine, Oregon Health Sciences University, Mail Code OP23, 3181 SW Sam Jackson Park Rd., Portland, OR 97201; Phone: 503-494-8464; E-mail: kamorton{at}earthlink.net.