Visual Abstract
Abstract
Fibroblast activation protein is a promising target for oncologic molecular imaging with radiolabeled fibroblast activation protein inhibitors (FAPI) in a large variety of cancers. However, there are yet no published recommendations on how to set up an optimal imaging protocol for FAPI PET/CT. It is important to optimize the acquisition duration and strive toward an acquisition that is sufficiently short while simultaneously providing sufficient image quality to ensure a reliable diagnosis. The aim of this study was to evaluate the feasibility of reducing the acquisition duration of [68Ga]FAPI-46 imaging while maintaining satisfactory image quality, with certainty that the radiologist’s ability to make a clinical diagnosis would not be affected. Methods: [68Ga]FAPI-46 PET/CT imaging was performed on 10 patients scheduled for surgical resection of suspected pancreatic cancer, 60 min after administration of 3.6 ± 0.2 MBq/kg. The acquisition time was 4 min/bed position, and the raw PET data were statistically truncated and reconstructed to represent images with an acquisition duration of 1, 2, and 3 min/bed position, additional to the reference images of 4 min/bed position. Four image quality criteria that focused on the ability to distinguish specific anatomic details, as well as perceived image noise and overall image quality, were scored on a 4-point Likert scale and analyzed with mixed-effects ordinal logistic regression. Results: A trend toward increasing image quality scores with increasing acquisition duration was observed for all criteria. For the overall image quality, there was no significant difference between 3 and 4 min/bed position, whereas 1 and 2 min/bed position were rated significantly (P < 0.05) lower than 4 min/bed position. For the other criteria, all images with a reduced acquisition duration were rated significantly inferior to images obtained at 4 min/bed position. Conclusion: The acquisition duration can be reduced from 4 to 3 min/bed position while maintaining satisfactory image quality. Reducing the acquisition duration to 2 min/bed position or lower is not recommended since it results in inferior-quality images so noisy that clinical interpretation is significantly disrupted.
The cancer incidence rate is increasing worldwide, and the global cancer burden is projected to double by 2040, with more than 28 million new cases each year (1). Access to diagnostic PET imaging will therefore need to be expanded. Apart from introducing new tracers, increasing access to imaging equipment, and providing continuous training of qualified personnel to tackle the rising incidence of cancer, other measures will also be needed. These include optimization of radiotracer activity, radiotracer accumulation, and acquisition duration to increase throughput without compromising image quality and ensuring that absorbed doses are kept as low as reasonably achievable.
Fibroblast activation protein is rapidly evolving as a promising target for oncologic molecular imaging in a large variety of cancers, as it is overexpressed by cancer-associated fibroblasts in 90% of all epithelial carcinomas (2). Several studies have shown the diagnostic potential of PET with fibroblast activation protein inhibitors (FAPI) (3) for detection and differentiation of primary tumors and metastases. At the time of writing (November 21, 2023), 90 clinical trials evaluating FAPI PET are registered on clinicaltrials.gov. However, most published studies of [68Ga]FAPI-46 (4–7) report a variety of acquisition durations, accumulation times, and administered activities, and there are no published guidelines on how to set up an optimal imaging protocol for FAPI imaging. The feasibility of making static acquisitions at different time points has been investigated. In a recent publication, Naeimi et al. evaluated [68Ga]FAPI-46 uptake at 3 different time points after administration in a variety of tumor types, revealing nonsignificant differences and suggesting the possibility of performing scans within 10–20 min after injection (8). Likewise, Ferdinandus et al. found that early imaging, 10 min after injection, resulted in lesion uptake and tumor detection equivalent to those of late imaging, 60 min after injection, for a heterogeneous group of cancers (5). Glatting et al. found that the biodistribution of different [68Ga]FAPI ligands had a significantly different dependence on imaging time. Therefore, the imaging time should be selected carefully, depending on the clinical setting (9). Glatting et al. also showed a superior target-to-background ratio for [68Ga]FAPI-46 compared with [68Ga]FAPI-02 and [68Ga]FAPI-74, which is consistent with results reported by Loktev et al. (10).
It is important to optimize acquisition duration, ensuring that image quality is good enough to ensure a reliable diagnosis. Visual grading using well-defined image quality criteria can be a useful tool when performing this type of optimization. For diagnostic radiographic images, there are guidelines regarding image quality criteria to be used when optimizing image quality (11). For nuclear medicine images, there are no predefined guidelines or recommendations to choose from. Hence, there is a need to determine well-defined criteria that are relevant for the specific radiotracer and anatomy being assessed.
This study aimed to evaluate the feasibility of reducing acquisition duration in [68Ga]FAPI-46 imaging while maintaining satisfactory image quality, with certainty that the radiologist’s ability to make a clinical diagnosis will not be affected.
MATERIALS AND METHODS
Study Design and Patient Cohort
This was a study of image quality in relation to acquisition duration and administered activity. The dataset was based on patients enrolled in an ongoing prospective clinical trial (NCT05172310, EudraCT 2020-002568-30) described in our previous publication (12). In brief, patients scheduled for surgical resection of suspected pancreatic and periampullary tumors underwent [68Ga]FAPI-46 PET/CT imaging before surgery. The Swedish Ethical Review Authority approved this study, and all subjects gave written informed consent.
Radiosynthesis and Image Acquisition
The FAPI-46 precursor was acquired from Sofie Biosciences, and the [68Ga]FAPI-46 was radiosynthesized on an Eckert & Ziegler Modular-Lab PharmTracer synthesis module, using 68GaCl3 eluate from a 68Ge/68Ga generator (13). Each patient was administered 3.6 MBq/kg ± 10%, if possible, dependent on body weight and labeling yield (minimum, 50 MBq; maximum, 370 MBq). Images were acquired on either a Discovery MI scanner (GE Healthcare; camera 1) or a Biograph mCT scanner (Siemens; camera 2) 60 min after injection. PET/CT imaging was performed from scull vertex to mid thigh, in step-and-shoot mode with list-mode enabled. The acquisition time was 4 min/bed position. Raw PET data were statistically truncated and reconstructed to represent images with 25%, 50%, and 75% of the acquisition duration, in addition to the 100% acquisition. Non–contrast-enhanced CT was performed for attenuation correction, and contrast-enhanced CT was performed directly after the PET scan. PET images were corrected for scatter, randoms, decay, and dead time and were reconstructed with ordered-subset expectation maximization, time of flight, and resolution recovery. The PET reconstruction parameters on the camera 2 were 2 iterations and 21 subsets with a matrix size of 200 × 200 and a gaussian postreconstruction filter of 5.0 mm in full width at half maximum. PET images from camera 1 were reconstructed with 8 iterations and 4 subsets, a matrix size of 256 × 256, a gaussian postreconstruction filter of 3.5 mm in full width at half maximum in the transaxial direction, and a 3-point convolution filter (1 mm in the x- and y-directions and 3.9 mm in the z-direction) in the axial direction. The axial field of view for cameras 2 and 1 were 21.6 and 20.0 cm, respectively. The reconstruction parameters were carefully selected to ensure equivalent (within ±10% variation) SUV and contrast in a PET body phantom with spheres.
Image Analysis and Quality Evaluation
We defined 4 image quality evaluation criteria by consensus (Table 1). Two criteria focused on the ability to distinguish specific anatomic details. The other two regarded perceived noise and overall image quality. Four sets of images were included for each patient, corresponding to the 4 different administered activities. These images were anonymized, randomized, and transferred to dedicated review software (Hermes Hybrid Viewer PDR, version 6.1.3; Hermes Medical Solutions AB). The intensity threshold was set to an SUV of 7.0. Image quality was evaluated by 2 board-certified radiologists (raters 1 and 2), one of whom was a board-certified nuclear medicine specialist and the other a specialist in training. Both readers had more than 2 y of clinical experience in diagnostic PET/CT imaging and about 2 y of experience in FAPI PET image evaluation. Images were scored on a Likert scale from 1 to 4. A score of 1 corresponded to complete certainty that the images did not fulfill the criteria; 2, near certainty that the criteria were not fulfilled; 3, near certainty that the criteria were fulfilled; and 4, complete certainty that the criteria were fulfilled.
Image Quality Criteria
Statistical Analysis
Visual Grading
To test the significance of differences in representations of acquisition duration, the data were analyzed within the visual grading regression framework by applying an ordinal logistic regression model (14). In this case, the model included 3 categoric fixed effects (acquisition duration in min/bed position, observer, and type of camera) and 1 random effect (patient) (15). The reference categories were 4 min/bed position (corresponding to 100% acquisition duration), camera 1, and rater 1. The calculations were performed in R (version 4.2.2), using the clmm function in the ordinal package. A P value less than 0.05 was considered statistically significant.
Objective Measurements
The primary lesions were defined using a 40% threshold isocontouring for all patients together with background volumes of interest in the Hermes Hybrid Viewer PDR. For each primary lesion, SUVmean and SUVmax were calculated. For each patient, background measurements were made in 3 separate locations that had homogeneous tracer uptake. The background in the liver was measured using a sphere with a diameter of 3 cm. A sphere with a diameter of 2 cm was used to measure the background in the ascending aorta, and a 3-cm sphere was placed in the musculature of the right thigh. Lesion-to-background ratio (LBR) was calculated for all patients and all acquisition durations using background measurements in the liver, aorta, and musculature. The coefficient of variance (COV), defined as the SD divided by the SUVmean, was calculated for all patients in the background liver, aorta, and musculature. SUV, LBR, and COV measurements were compared using the paired Wilcoxon test. A P value of less than 0.05 was considered statistically significant. The quantitative measurements were analyzed using SPSS Statistics (version 25; IBM) for Windows (Microsoft).
RESULTS
Patient Cohort and Imaging Acquisition
Among the subjects enrolled in the ongoing clinical trial, the first 10 patients (6 men and 4 women; mean age, 69.9 ± 11.3 y; range, 49–85 y) to receive an administered activity of 3.6 MBq/kg ± 10% were selected for the study. The mean total injected activity was 254.9 ± 25.8 MBq (range, 213.2–292.1 MBq), and the mean injected activity by body weight was 3.6 ± 0.2 MBq (range, 3.4– 4.0 MBq/kg). The statistically truncated images, representing images with 25%, 50%, 75%, and 100% of the acquisition duration, resulted in 4 sets of images corresponding to an acquisition duration of 1, 2, 3, and 4 min/bed position, respectively. The mean accumulation time was 60.5 ± 3.5 min (range, 56–67 min). The clinical characteristics of the patients are presented in Table 2.
Patient Characteristics
Image Analysis and Quality Evaluation
Visual Grading
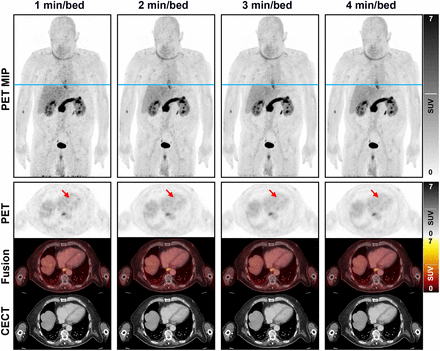
Images corresponding to the 4 acquisition durations in a 74-y-old man with histologically confirmed ductal adenocarcinoma in the head of the pancreas (patient 6) are shown in Figure 1. A graphical presentation of the visual grading results is given in Figure 2.
Maximum-intensity projection and axial PET images with corresponding contrast-enhanced CT and fused images presenting image quality in relation to acquisition duration of [68Ga]FAPI-46 imaging. Reference line (blue) for axial images is presented on maximum-intensity projection images. Arrows indicate interventricular septum of heart (image quality criterion B). CECT = contrast-enhanced CT; MIP = maximum-intensity projection.
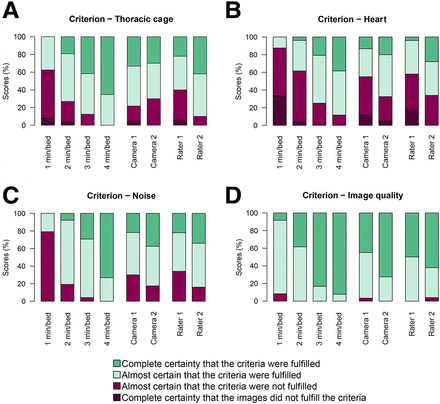
Results from scoring of criteria A–D presented with histogram for each camera, each rater, and each acquisition duration.
The graphs show, for all criteria, a visual trend toward increasing image quality scores with increasing acquisition duration. Formal testing of these patterns is found in Table 3, where positive regression coefficients represent higher image quality scores than the reference categories (4 min/bed position, camera 1, and rater 1) and P values refer to significance tests relative to these reference categories. For all criteria, the 2 shortest acquisition durations had scores that were lower than the reference level to a strongly significant extent. The images acquired at 3 min/bed position had significantly lower values for criteria A (thoracic cage), B (heart), and C (noise) but not for criterion D (overall image quality). The scores given by rater 2 were significantly higher than those given by rater 1 for criteria A, B, and C but not for overall image quality (criterion D). When the cameras were compared, camera 2 had significantly higher scores for criteria B, C, and D, whereas there was no significant difference for criterion A.
Analysis of Image Quality Scoring Using Visual Grading Regression
The proportion of images for which the rater was certain or almost certain (score 3 or 4) that the image quality criterion was fulfilled at 3 min/bed position was 85% for criterion A, 75% for criterion B, 95% for criterion C, and 100% for criterion D (Fig. 2). For the shorter acquisition durations (1 and 2 min/bed position), the corresponding proportion was considerably lower, except for overall image quality (criterion D), for which the proportion remained at or above 90%.
Objective Measurements
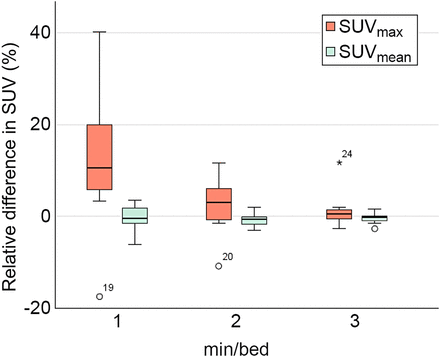
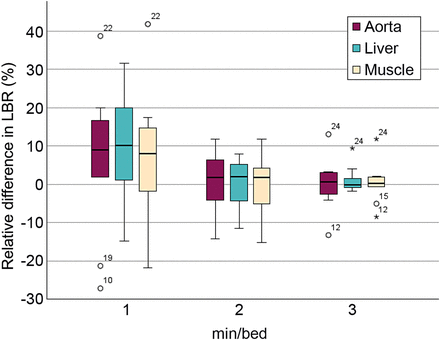
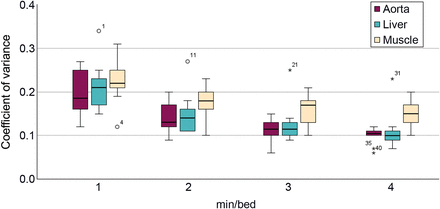
The difference in SUVmax and SUVmean for 1, 2, and 3 min/bed position relative to 4 min/bed position is presented in Figure 3. For SUVmax, a trend toward decreasing range and median value relative to 4 min/bed position with an increase in acquisition duration was observed. For SUVmean, the range decreased when the acquisition duration increased, but the median value did not change significantly. The results from the paired Wilcoxon signed rank test performed on the objective measurements are presented in Table 4. The only significant difference was for SUVmax when 1 min/bed position was compared with 4 min/bed position (z = −1.99, P < 0.05). The relative difference in LBR for each acquisition duration relative to 4 min/bed position using the background liver, aorta, and musculature is presented in Figure 4. When assessing the results of the relative difference in LBR compared with 4 min/bed position, a trend toward decreasing range and median value with an increase in acquisition duration was observed for all physiologic uptakes that were used as background. In all COV measurements, the median COV decreased with an increase in acquisition duration. The difference was significant between 4 min/bed position and all other acquisition durations for the measurements made in the background liver, aorta, and musculature (Table 4). The measured COV in the background liver, aorta, and musculature of the right thigh is presented for each acquisition duration in Figure 5.
Box plots of differences in SUVmax and SUVmean for 1, 2, and 3 min/bed position relative to 4 min/bed position. Asterisk represents outlier plotted as individual point.
Comparison of SUVmax, SUVmean, and COV Measurements Using Paired Wilcoxon Signed-Rank Test
Box plots of LBR relative to 4 min/bed position using background liver, aorta, and musculature. Asterisks represent outliers plotted as individual points.
Box plots of COV measured in background liver, aorta, and musculature at 1, 2, 3, and 4 min/bed position. Asterisks represent outliers plotted as individual points.
All measurements of SUVmax, SUVmean, LBR, and COV are presented in Supplemental Table 1 (supplemental materials are available at http://jnmt.snmjournals.org). The mean, range, and percentiles from the box plots are presented in Supplemental Table 2.
DISCUSSION
As the incidence of cancer has been gradually increasing worldwide in recent years (16), the clinical need for diagnostic PET imaging has been growing. With several new molecular PET imaging agents being developed, and with theranostics reemerging in the era of personalized medicine, a lean workflow and optimal imaging protocols are becoming more and more important for nuclear medicine clinics to meet the rising demand. To the best of our knowledge, no publication has investigated the possibility of optimizing acquisition duration or administered activity in static FAPI images, for any of the available FAPI ligands, acquired 1 h after injection. The aim of this study was to evaluate the optimal acquisition duration for [68Ga]FAPI-46 on the basis of image quality assessments. The visual grading regression showed no significant decrease in overall image quality when the acquisition duration was reduced by 25% from 4 to 3 min/bed position.
When the acquisition duration was reduced by 25%, there was a significant decrease in image quality scores for criteria A, B, and C. Still, as can be seen in Figure 2, the raters were certain or almost certain that the image quality was sufficient in 75%–100% of cases for all criteria. It may be argued that these proportions are more relevant than the presence or absence of statistically significant differences between the 2 highest activity levels. A possible conclusion is that a reduction in acquisition duration by 25% from the current standard might be feasible, with acceptable impairment of image quality. On the other hand, any further reduction in acquisition duration seems to be associated with a more dramatic decrease in image quality, with less than half the scores being 3 or 4 for criterion B (Fig. 2).
Criteria A and B, which focused on visualization of well-defined anatomic structures, tended to have lower image quality scores than the more general concepts of image noise (criterion C) and overall quality (criterion D), in particular at shorter acquisition durations. A possible reason is that any interference in clinical image interpretation brought about by reducing the acquisition duration is more likely to be caused by inferior anatomic visualization than by the associated increase in image noise level. A possible general conclusion from these results is that the anatomic criteria were well formulated to study how an increase in acquisition duration improved visualization of anatomic structures.
An interesting observation is that for 3 of 4 criteria, camera 2 had significantly higher image quality scores than camera 1 (Table 3). This result was unexpected, since the reconstruction parameters were carefully selected to ensure a harmonized contrast and SUV, as 4 patients were scanned on camera 2 and 6 patients on camera 1 (Table 2). The reason for the difference in rating does not seem to stem from a difference in body mass index between patients, as they were similar (average, 22.8 for camera 1 vs. 24.3 for camera 2). Likewise, the mean age of the patients was similar (72 y for camera 1 vs. 77 y for camera 2). However, physiologic uptake of [68Ga]FAPI-46 can vary considerably between patients, as observed in several previous studies (12,17), and considering the relatively small number of patients included in this study, this variation could be an explanation. Despite this observation, the finding of increasing image quality scores with increasing acquisition duration was similar for both cameras.
As expected, our measurements of SUVmean remained relatively stable when the acquisition duration was decreased. SUVmax increased when acquisition duration decreased, which is an expected result since SUVmax is a semiquantitative measurement known to be sensitive to the presence of increased noise in the images. Even though the results were statistically significant only when 1 min/bed position was compared with 4 min/bed position, a large increase in the range and mean value was observed relative to 4 min/bed position when the acquisition duration was reduced (Supplemental Table 2).
The variation in LBR showed a similar pattern to the SUVmax measurements regardless of which physiologic uptake was used as background; this result is expected since these measurements per definition are based on the SUVmax measurements. The COV measurement, which is used as a measure to quantify noise, illustrates how a decrease in acquisition duration results in increased noise in the images. This is consistent with the observations from the visual grading evaluation, in which images with a shorter acquisition duration lowered image quality scores.
To our knowledge, this study was the first to evaluate the effect of [68Ga]FAPI-46 acquisition duration on image quality. When evaluating the radiation dosimetry and biodistribution of [68Ga]FAPI-46 in cancer patients, Meyer et al. administered an activity in the range of 100–370 MBq per examination using a continuous-bed-motion acquisition technique at 0.7 mm/s (4). The same image acquisition technique with comparable injected activity ranges was reported in several other publications, such as Loktev et al. (range, 216–242 MBq) (10), Koerber et al. (range, 111–298 MBq) (18), and Röhrich et al. (range, 167–293 MBq) (19) when assessing the impact of [68Ga]FAPI-46 PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. Glatting et al. administered activities in the range of 150–250 MBq, using the same acquisition technique but with the continuous bed motion set to 1.6 mm/s (20). Dendl et al. reported an injected activity in the range of 52–325 MBq with step-and-shoot acquisition and times of 3–5 min/bed position (21). Mona et al. administered a set amount of activity (range, 174–185 MBq), adjusting the acquisition time per bed position (2–4 min/bed position) depending on patient body weight (7). Kessler et al. reported an injected activity range of 144 ± 36 MBq but did not mention the acquisition time (22). None of these publications provided activities per patient body weight, and image acquisition techniques varied substantially. Furthermore, although satisfactory image contrast with high LBRs was reported in some instances, no specific evaluation of image quality was performed, making any kind of direct comparison with our results infeasible.
In summary, a reduction of acquisition duration to 1 min/bed position was ruled out because of the low scores for criteria A, B and C. Choosing between a reduction to 2 min/bed position and a reduction to 3 min/bed position is less straightforward. The scores were lower for 2 min/bed position than for 3 min/bed position for all criteria (Fig. 2). However, when the acquisition duration was reduced to 2 min/bed position, fewer than half the patients received a satisfactory score (3 or 4) for criterion B, preventing us from advocating such a large reduction. Thus, we settled for suggesting 3 min/bed position as the recommended acquisition duration rather than 2 min/bed position.
An important matter to address is that the calibration factor for 68Ga on the dose calibrator was adjusted to increase the accuracy of 68Ga quantification after the 10 patients included in this study had their examinations performed. The change in calibration factor led to a decrease in measured activity by 6.1%. Thus, all examinations performed with the old calibration factor had to be corrected retrospectively. The corrected administered activities are presented in this paper. The problem with the selection of an accurate calibration factor for 68Ga has been highlighted in studies by Bailey et al. and Sanderson et al. (23,24). A limitation of this study was the relatively small sample size, and conclusions should therefore be drawn with caution. Furthermore, only patients with pancreatic and periampullary cancers were included, and even though the sampling was systematic, it might not have been representative of the general oncologic population. Another limitation is that SUV and contrast had to be harmonized between the cameras since the purpose of the ongoing clinical trial at our department is to determine the diagnostic accuracy of [68Ga]FAPI-46 PET/CT in patients with various cancer types (NCT05172310). The need for harmonization put constraints on the freedom with which we could optimize the reconstruction parameters on each camera separately. It is also important to consider that the conclusion drawn from this study is limited to cameras that have similar performance in terms of, for example, sensitivity. Lastly, the optimization was made on list-mode data that were statistically truncated. An optimization performed on this type of dataset relies on the assumption that statistically truncated PET images accurately represent patient images acquired with a shorter acquisition duration. When the detector dead time can be neglected, the number of true and scattered coincidences scales linearly with the administered activity. Random coincidences scale with the square of the administered activity (25). Therefore, truncated data contain a higher random fraction than one would expect from a corresponding reduction in administered activity or acquisition time, resulting in increased noise in the truncated images. In theory, this would make the conclusion drawn from this study, that is, the feasibility of reducing the acquisition duration by 25%, a conservative conclusion, since images with a reduced acquisition duration of 25% would contain less noise than the truncated images. As an alternative, it would also be feasible to decrease the administered activity rather than the acquisition duration while maintaining the same counting statistics in the images. Schaefferkoetter et al. performed a study in which this assumption was validated, comparing truncated standard-activity images with actual low-activity images (26). Several other optimization studies using truncated PET data have been performed on 18F-FDG images (27–29).
CONCLUSION
Our study showed that for [68Ga]FAPI-46 PET imaging of oncologic patients with an administered activity of 3.6 MBq/kg, satisfactory image quality could be maintained when the acquisition duration was reduced from 4 to 3 min/bed position, although objective differences in certain specific metrics of image quality could be observed. A reduction of the acquisition duration to 2 min/bed position or lower is, however, not recommended since it results in images of unacceptably low quality and produces noise that significantly disrupts clinical interpretation. An optimized examination protocol with a shortened acquisition duration may result in an increased production output and leaner workflow at the clinic. Alternatively, an equivalently reduced amount of administered activity would ensure a justified and as-low-as-reasonably-achievable radiation dose to the patients. An increase in the number of examinations per batch of radiotracer would concomitantly improve cost efficiency. Confirmation of these findings in a larger cohort will be required to validate our results.
DISCLOSURE
The study was financed by grants from the Swedish government under the ALF agreement (SLL20200025) and from the Swedish Cancer Society (200695). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can the acquisition duration of [68Ga]FAPI-46 be decreased while still maintaining satisfactory diagnostic image quality?
PERTINENT FINDINGS: The acquisition duration can be reduced from 4 to 3 min/bed position while maintaining satisfactory image quality, with an administered activity of 3.6 MBq/kg. Further reducing acquisition duration is inadvisable, as it increases noise and degrades image quality, adversely affecting clinical interpretation.
IMPLICATIONS FOR PATIENT CARE: The recommended shortened acquisition duration could enable more patient examinations, increasing diagnostic capacity. Alternatively, an equivalent reduction in administered activity would ensure a justifiable and as-low-as-reasonably-achievable dose to the patients.
Footnotes
↵* Contributed equally to this work.
Published online Apr. 16, 2024.
REFERENCES
- Received for publication November 28, 2023.
- Revision received March 4, 2024.