Abstract
Our objective was to determine the stability of stabilized 99mTc-hexamethylpropylene amine oxime (99mTc-d,l-HMPAO) dispensed by vial and syringe, with the storage time and labeling activity varied. Methods: 99mTc-d,l-HMPAO was labeled according to the manufacturer's instructions, but with modification of the 99mTcO4Na activity. Two groups were prepared: 1,110 MBq (30 mCi) and 2,600–3,700 MBq (70.3–100 mCi). Five minutes after labeling, the radiochemical purity (RCP) of the vial content was determined. Afterward, the same activity was distributed into two 2-mL syringes and into the manufacturer's vial. In one of the syringes, the radiopharmaceutical stayed in contact with the needle for 4 h. At 2 and 4 h after labeling, the RCP of the vial and syringe content was checked and compared. Results: The mean RCP of stabilized 99mTc-d,l-HMPAO labeled with 1,110 MBq (30 mCi) and stored in a vial decreased from 93.1% at 5 min to 92.1% at 2 h and to 91.1% at 4 h. With storage in a syringe, the RCP decreased from 89.8% at 2 h to 88.7% at 4 h. This diminution increased for labeling with higher activities (2,600–3,700 MBq [70.3–100 mCi]), ranging from 91.4% at 5 min, 89.0% at 2 h, and 85.3% at 4 h in a vial and from 85.9% at 2 h to 80.2% in a syringe. 99mTcO2 and secondary 99mTc-HMPAO were the main impurities at t = 0. 99mTcO4− was an impurity that increased with time in both vials and syringes but significantly so in syringes. All these impurities were higher with labeling activities in the range of 2,600–3,700 MBq (70.3–100 mCi). Contact of the needle with 99mTc-d,l-HMPAO sharply decreased the RCP to 57.1% at 4 h. Conclusion: The RCP of stabilized 99mTc-d,l-HMPAO decreases significantly in both vials and syringes with high labeling activities. The product is less stable when stored in a syringe than in a vial. The fraction of dose in contact with the needle affects the RCP results.
Hexamethylpropylene amine oxime labeled with 99mTc (99mTc-d,l-HMPAO) is a radiopharmaceutical used in regional cerebral perfusion studies (1). d,l-HMPAO is the active principal of the specialty pharmaceutical sold under the name Ceretec (GE Healthcare Bio-Sciences) in the form of a freeze-dried vial for reconstitution with sodium pertechnetate (99mTcO4Na). The stereoisomer 99mTc-d,l-HMPAO is a neutral and lipophilic complex able to cross the intact blood–brain barrier. Once in the brain, it experiences a transformation mediated by glutathione (2,3), becoming a less lipophilic complex. This complex is unable to recross the blood–brain barrier and remains retained in the brain, allowing the tomographic study.
The reconstitution of a freeze-dried d,l-HMPAO vial with technetium generates 3 forms of radiochemical impurities: secondary 99mTc-HMPAO, free technetium (99mTcO4), and reduced hydrolyzed technetium (99mTcO2). The ratio between them varies rapidly with time according to labeling conditions (4), determining the radiopharmaceutical stability. This parameter limits the 99mTc-d,l-HMPAO administration to 30 min after labeling (5).
The manufacturer recommends that the RCP of this radiopharmaceutical be at least 80%. Actually, an RCP of at least 85% is needed to obtain a high-quality image (6,7).
There are different methods of stabilizing 99mTc-d,l-HMPAO: addition of gentisic acid (8), preparation of the radiopharmaceutical in ethanol (9,10), and addition of methylene blue (11) or cobalt chloride (11–14). Of these methods, the addition of cobalt chloride after labeling has shown the best results. This form of stabilization had led to the commercialization of another specialty pharmaceutical, named stabilized Ceretec, which is stable for 6 h after labeling (15) according to the manufacturer.
This specialty pharmaceutical can be prepared in centralized units far from where it is going to be administered. Because stabilized 99mTc-d,l-HMPAO is prepared several hours before its administration, 2 variables can potentially influence its stability: the labeling activity and the length of storage time in plastic syringes (16).
We occasionally have noticed some problems in the interpretation of perfusion images obtained with monodoses supplied in plastic syringes by radiopharmacy centralized units. We have observed a rise in the background and extracerebral activities related to cortical activity. In all cases, the RCP proved to be less than 85%. Variability in extracerebral activity has been attributed to a decrease in RCP that has occasionally been described when labeling activity for 99mTc-d,l-HMPAO exceeds the specifications of the manufacturer (7). It is also known that stabilized 99mTc-d,l-HMPAO is less stable when stored in syringes than in vials (16).
Our objective was to determine the stability of stabilized 99mTc-d,l-HMPAO dispensed by vials and syringes, according to the storage time and the labeling activity.
MATERIALS AND METHODS
We prepared 10 vials of stabilized Ceretec labeled with the maximum activity recommended: 1,110 MBq (30 mCi) of 99mTcO4Na (15). Another 10 vials were labeled with higher activities: 2,600–3,700 MBq (70.3–100 mCi). All manufacturer specifications (except activity) were followed in all 20 preparations.
The RCP of 99mTcO4Na was determined by instant thin-layer chromatography using silica gel strips as a support and butan-2-one as a solvent.
We studied the stability of stabilized 99mTc-d,l-HMPAO in the vial (where it is originally supplied) and in the plastic syringe (Becton-Dickinson) where it is stored from the moment of its preparation in the radiopharmacy centralized unit until it is administered.
Immediately after the RCP of the vial had been determined, the total activity was equally distributed among the vial and two 2-mL plastic syringes, and the vial and syringes were stored at room temperature. The RCP of the vial and syringes was also determined after 2 and 4 h.
To determine the influence of the needle on the RCP of the stabilized 99mTc-d,l-HMPAO, in one of the syringes the radiopharmaceutical was never allowed to contact the needle. In the other syringe, stabilized 99mTc-d,l-HMPAO was in contact with the needle (19-gauge; 8.9 cm [3.5 in] long) from the beginning to the end of the study.
The RCP was assessed using 2 strips of instant thin-layer chromatography silica gel as a support and butan-2-one as a solvent in the first chromatogram to quantify secondary 99mTc-HMPAO and 99mTcO2. Sodium chloride (0.9%) was the solvent used in the second chromatogram to quantify 99mTcO4−. The activity in the strips was determined with a miniGITA radiochromatography scanner (Raytest).
The Student t test was used to compare results between the mean value of each group. A P value of less than 0.05 was considered statistically significant.
RESULTS
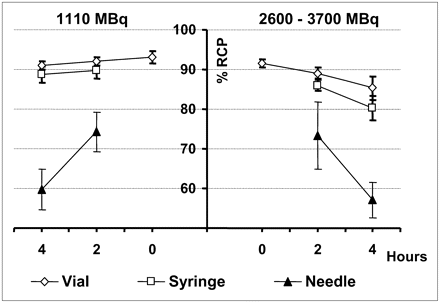
The RCP of the 99mTcO4Na used in the labeling was 97.8 ± 1.5. The RCP of the stabilized 99mTc-d,l-HMPAO showed significant differences between vials and syringes for both groups of labeling activities at all times studied. But the RCP decreased significantly (P < 0.01) in vials and in syringes as labeling activity increased (Table 1). This diminution was more important in syringes, resulting in values of 80.2% after 4 h in the high-activity group (Table 1; Fig. 1). This RCP value was in the limit permitted for administration according to the manufacturer. The RCP in vials remained higher than 85% for both groups of activities at 2 and 4 h.
Percentage of RCP of stabilized 99mTc-d,l-HMPAO over time in both groups of labeling activities and forms of storage.
Effect of Labeling Activity and Storage Form on RCP of Stabilized 99mTc-d,l-HMPAO over Time
The solution of stabilized 99mTc-d,l-HMPAO in contact with the needle was unstable, and the RCP dropped to values lower than 80% at 2 h and lower than 60% after 4 h (Table 1). Accordingly, to correctly determine the RCP of the stabilized 99mTc-d,l-HMPAO, one should discard the radiopharmaceutical solution in contact with the needle.
The initial RCP of the stabilized 99mTc-d,l-HMPAO in vials was higher than 90% (Fig. 1). 99mTcO2 and secondary 99mTc-HMPAO were the main impurities (Fig. 2). The quantity of impurities formed at 2 and 4 h in vials and syringes for both groups of activities is detailed in Table 2.
Percentage of radiochemical impurities in stabilized 99mTc-d,l-HMPAO over time in vials and syringes (mean ± SD; n = 10).
Effect of Labeling Activity and Storage Form on Radiochemical Impurities in Stabilized 99mTc-d,l-HMPAO in Vials and Syringes over Time
Quantities of 99mTcO2 and secondary 99mTc-HMPAO were significantly higher when the vials were labeled with activities of 2,600–3,700 MBq. In contrast, low quantities of 99mTcO4− were found at t = 0 for both groups of activity. 99m TcO4− increased with time in vials and in syringes, being significantly higher (P < 0.01) in syringes at 2 and 4 h for both groups of activities (Table 2; Fig. 2).
We also observed that the value of RCP obtained from the fraction of the dose in contact with the needle decreased significantly with time independently of the labeling activity (Fig. 1).
DISCUSSION
Stabilized 99mTc-d,l-HMPAO as prepared by the hospital radiopharmacy is intended to be injected immediately. The radiopharmaceutical is stored in a vial, and the time of storage in a syringe is minimal. In contrast, radiopharmacy centralized units prepare the dose several hours before it is administered in the nuclear medicine department, implying the use of higher labeling activities and longer storage in a syringe, which is the dosage form provided by these centralized units.
We have found that cerebral perfusion studies performed with stabilized 99mTc-d,l-HMPAO supplied in a syringe by the radiopharmacy centralized unit have occasionally shown a decrease in relative cerebral cortical uptake because of an increase in background activity. This problem poses some difficulties when interpreting the images.
The aim of this study was to determine whether the stability of stabilized 99mTc-d,l-HMPAO is affected by the amount of labeling activity and by the storage form (vial or syringe).
After determining the results of this study, we have observed that the RCP of stabilized 99mTc-d,l-HMPAO decreases both in vials and in syringes when labeling activity increases. This instability is higher in syringes (16): when the labeling activity is in the range of 2,600–3,700 MBq (70.3–100 mCi), the RCP after 4 h is 80%. This value is within the limit for administration, with 99mTcO4− being the main impurity. Obviously, stabilized 99mTc-d,l-HMPAO labeled with a high activity and stored in a syringe cannot, at 6 h, attain the RCP recommended by the manufacturer (15). Some authors report that RCP values of at least 85% must be attained to obtain a good-quality image (6,7). The increase of 99mTcO4− may be due to the increased rate of reoxidation of stabilized 99mTc-d,l-HMPAO to 99mTcO4− (16). It is unclear if reoxidation is related to the material constituting the container (plastic syringe) or to air in the product.
Stability can possibly be improved by enhancing the manufacturer-established conditions of the technetium elution used for the labeling. Ballinger et al. (7) showed that the RCP (time = 0) of 99mTc-d,l-HMPAO was not affected by the effect of generator ingrowth or by the time elapsed from elution to labeling. Nevertheless, they did not determine the RCP at different times after labeling but instead only the initial RCP. This fact may relate to our finding that 99mTcO4− is initially low and then gradually increases.
The radiopharmacy unit that makes the preparation must look for possible improvements to obtain a final RCP of at least 80% at the time of administration. Among these improvements are optimizing the elution quality, choosing the vial as the dosage form, and, if necessary, reducing the time between labeling and administration.
RCP must be determined from a solution of stabilized 99mTc-d,l-HMPAO that has not been in contact with the needle. The instability of stabilized 99mTc-d,l-HMPAO in contact with a needle, compared with that in a vial or syringe, is probably related to metallic compounds.
CONCLUSION
The RCP of stabilized 99mTc-d,l-HMPAO decreases in both vials and syringes when labeling activities higher than 2,600 MBq (70.3 mCi) are used. The stabilized 99mTc-d,l-HMPAO is more unstable in syringes than in vials. When high activities are used, the syringe as a dosage form should be rejected, specially if storage time exceeds 2 h. The fraction of the dose in contact with the needle should be discarded in the determination of RCP.
Footnotes
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- Received for publication April 17, 2008.
- Accepted for publication August 20, 2008.