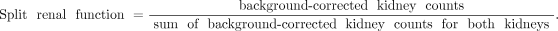RATIONALE
Cortical renal imaging has a high diagnostic sensitivity (>90%) for detecting renal cortical defects. Blood is filtered within the kidney nephrons, specifically the glomerulus of the nephron, located within the renal cortex. Defects seen on renal cortical imaging indicate a loss of function, most commonly due to pyelonephritis or scarring.
CLINICAL INDICATIONS
Detection of permanent renal parenchymal scarring.
Detection of acute pyelonephritis.
Detection of parenchymal damage after trauma.
Characterization of structural renal abnormalities: for example, solitary kidney, duplex kidney, small kidney, dysplastic kidney, and horseshoe kidney.
Detection of ectopic renal tissue, including cross-fused renal ectopia.
Quantitation of differential (split) renal function.
Confirmation of nonfunctional multicystic dysplastic kidney.
Evaluation of unexplained hypertension when there is clinical suspicion of renal disease such as dysplasia or scarring.
Evaluation of renal parenchymal function in patients with renovascular hypertension before and after revascularization procedures.
Regional assessment of renal parenchymal function in patients with complex renal calculi before and after treatment.
Surgical decision-making for ureteropelvic junction obstruction or refractory vesicoureteral reflux based on differential renal function.
Evaluation of renal parenchyma when there is an allergy to iodinated CT contrast media and MRI is unavailable or contraindicated.
CONTRAINDICATIONS
Pregnancy or breastfeeding (pregnancy must be excluded according to local institutional policy; if the patient is breastfeeding, appropriate radiation safety instructions should be provided).
Recent nuclear medicine study (radiopharmaceutical-dependent).
PATIENT PREPARATION/EDUCATION
The patient may eat and take medications as necessary before the procedure. Increased hydration is not required for a 99mTc-dimercaptosuccinic acid renal cortical scan as it is for other renal imaging.
A focused past medical history containing the following elements should be obtained:
○ Diabetes.
○ Hypertension.
○ Kidney disease: urinary tract infections, calculi, pyelonephritis.
○ Autoimmune disease.
○ Signs and symptoms, including fever.
○ Current medications, including over-the-counter drugs.
○ Results of clinical laboratory tests.
○ Results of other diagnostic tests, including ultrasonography, MRI, and CT (including contrast administration).
○ Fluid input and output.
RADIOPHARMACEUTICAL IDENTITY, DOSE, AND ROUTE OF ADMINISTRATION
Radiopharmaceutical identity, dose, and route of administration are presented in Table 1.
Radiopharmaceutical Identity, Dose, and Route of Administration
ACQUISITION PARAMETERS: PLANAR
Planar acquisition parameter are presented in Table 2.
Acquisition Parameters: Planar
ACQUISITION PARAMETERS: SPECT OR SPECT/CT
SPECT or SPECT/CT acquisition parameters are presented in Table 3.
Acquisition Parameters: SPECT or SPECT/CT
PROTOCOL/ACQUISITION INSTRUCTIONS
Instruct the patient to void immediately before the procedure.
Image 2–4 h after radiopharmaceutical administration.
When using a low-energy, high-resolution collimator, position the patient supine on the imaging table with the camera in the posterior position, ensuring the kidneys are in the field of view. Perform posterior imaging to evaluate native kidneys and anterior imaging to evaluate transplanted kidneys or patients with a known or suspected horseshoe kidney.
Obtain the following static images: right posterior oblique, left posterior oblique, right lateral (optional), and left lateral (optional). An anterior image may also be acquired if a horseshoe kidney is suspected or for evaluation of a renal transplant. Each image is obtained by acquiring 500,000–1 million counts.
For pinhole imaging (pediatric patients), position the patient prone on the imaging table. The kidney should fill approximately 75% of the field of view for each pinhole image. The pinhole aperture–to–patient distance should be the same for all views.
If required in patients with impaired renal function, perform additional delayed imaging at 6–24 h.
Instruct the patient to hydrate and void frequently the rest of the day to minimize the radiation dose to the kidneys and bladder.
IMAGE PROCESSING
Draw a region of interest around each kidney and the aorta.
Draw 2 C-shaped perirenal background regions of interest (one for each kidney) on the posterior images. The background regions should be 2 pixels wide and 1 pixel away from the whole-kidney region.
Express the counts per kidney as a function of 100% total function. The normal contribution for each kidney is 45%–55%:

Scale planar images to visualize areas of normal anatomy and increased uptake accurately.
Reconstruct the SPECT data using filtered back projection or iterative reconstruction and display in the coronal, sagittal, and transverse projections.
Footnotes
Published online Aug. 13, 2024
REFERENCES
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.