The introduction of cardiac scintigraphy with bone-seeking tracers such as [99mTc]Tc-pyrophosphate as a highly accurate diagnostic test for transthyretin cardiac amyloidosis (CA) has been transformative for the field of CA by facilitating a noninvasive diagnosis and obviating cardiac biopsy. This review will describe the impact of [99mTc]Tc-pyrophosphate scintigraphy on the field, the evolution of the test, and its strengths and potential pitfalls.
CA is a systemic disease that occurs due to the deposition of aggregated misfolded protein in the myocardial interstitium (1). There are 2 major forms: transthyretin amyloidosis and light-chain amyloidosis, with clinical manifestations depending on the protein that is deposited (2). In light-chain amyloidosis, the amyloid fibrils are derived from excess immunoglobulin light chains, which are produced by clonal plasma cell disorders (3), and in transthyretin amyloidosis, the amyloid fibrils are formed from transthyretin, a protein synthesized in the liver with the function of transporting thyroid hormone and vitamin A (4). Transthyretin CA is further subdivided into wild-type and variant transthyretin amyloidosis, with the former being associated with age-related protein misfolding and the latter being an autosomal dominant substitution mutation in the TTR gene located on the long arm of chromosome 18. Approximately 120 pathogenic TTR gene mutations have been reported (5). The median survival from diagnosis, if left untreated, is less than 6 mo for light-chain CS (6) and 3–5 y for transthyretin CA (4). The marked differences in therapy and prognosis between light-chain and transthyretin amyloidosis make diagnostic mistakes costly and consequential. Thus, until recently, an endomyocardial biopsy was the only way to make a confirmed diagnosis of CA and its type. More recently, the ability to make a confident noninvasive diagnosis of CA has unmasked a previously unrecognized high prevalence of transthyretin CA in the general population (7). The confluence of this new diagnostic capability, the recognition of a high community prevalence, and the recent introduction of effective therapy for transthyretin CA have resulted in an unprecedented focus on this disease.
RISE IN DIAGNOSTIC IMAGING
CA is difficult to diagnose without imaging. Although several red-flag symptoms and signs are recognized (Table 1), none are diagnostic individually or in combination. However, consideration of these symptoms and signs is often helpful in constructing a clinical probability of CA and when interpreting equivocal imaging findings. The nonspecific nature of the symptoms and signs of CA invariably delays the diagnosis (8). With the recent increase in the awareness of CA, there has been a substantial increase in the referral of patients for diagnostic imaging (9,10). Many nuclear cardiology laboratories in the United States have begun to offer [99mTc]Tc-pyrophosphate imaging. With very little promotion of this test by the industry, [99mTc]Tc-pyrophosphate scintigraphy now comprises an increasing proportion of radionuclide-based imaging tests performed in the United States, a testament to its unique and niche capability.
CA Red Flags
BONE-SEEKING TRACERS USEFUL FOR DIAGNOSIS OF TRANSTHYRETIN CA
There is geographic variability in the availability of bone-seeking tracers that can be used for cardiac scintigraphy for transthyretin CA. In the United States, [99mTc]Tc-pyrophosphate is used most often for this purpose. [99mTc]Tc-hydroxymethylene diphosphonate is also available and can be used interchangeably with similar dose, protocol, and interpretation parameters (11). Most importantly, [99mTc]Tc-methyl diphosphonate, which is the most widely used bone tracer in the United States, is not sensitive for the diagnosis of transthyretin CA and should not be used for this purpose (2,12). In Europe, [99mTc]Tc-3,3-diphosphono-1,2-propanodicarboxylic acid ([99mTc]Tc-DPD) is the most commonly used bone-seeking tracer for the diagnosis of transthyretin CA. Given the geographically restricted availability of these tracers, comparative studies between [99mTc]Tc-pyrophosphate and [99mTc]Tc-DPD do not exist, and similarity between protocol and interpretation parameters must not be assumed.
EVOLUTION OF CARDIAC SCINTIGRAPHY WITH BONE-SEEKING TRACERS FOR TRANSTHYRETIN CA
Case reports and observational reports on the diagnostic utility of [99mTc]Tc-pyrophosphate in transthyretin CA date back to the late 1970s and early 1980s (13–15). These reports included patients with both transthyretin and light-chain CA and were published before the trophism of bone-seeking tracers for transthyretin CA, but not light-chain CA, was recognized. Thus, these early reports concluded that [99mTc]Tc-pyrophosphate scintigraphy has a poor sensitivity for the diagnosis of transthyretin CA. The publication of a seminal multinational paper from the U.K. National Amyloidosis Center in 2016 focused attention on the high diagnostic accuracy of [99mTc]Tc-DPD, [99mTc]Tc-pyrophosphate, and [99mTc]Tc-hydroxymethylene diphosphonate for the diagnosis of transthyretin CA when light-chain CA was excluded with concomitantly performed serum and urine studies (16). This study used planar imaging of [99mTc]Tc-pyrophosphate and semiquantitative grading of tracer uptake in the myocardium compared with the ribs, a scoring system proposed by Perugini et al. in 2005 (17). Indeed, most of the contemporary data that established the use of [99mTc]Tc-pyrophosphate and [99mTc]Tc-DPD in transthyretin CA is based on planar imaging using the Perugini score and a heart–to–contralateral-lung ratio introduced after the Perugini score (Fig. 1A) (18). However, increasing experience with [99mTc]Tc-pyrophosphate and [99mTc]Tc-DPD scintigraphy has exposed limitations of the planar imaging approach, which cannot distinguish between tracer in the left ventricle myocardium and blood pool (Fig. 1B). That limitation results in a high prevalence of false-positive [99mTc]Tc-pyrophosphate/DPD results. An expert recently reported that 64% of results are false-positive with planar interpretation alone (10). Thus, contemporary [99mTc]Tc-pyrophosphate and [99mTc]Tc-DPD interpretation must be based on the demonstration of tracer uptake in the myocardium by SPECT imaging. The Perugini grade and heart–to–contralateral-lung ratio may be used as supplementary information during interpretation but cannot be the primary diagnostic criteria. When available, the use of SPECT/CT systems or simultaneous dual-isotope 201Tl/[99mTc]Tc-pyrophosphate scintigraphy may improve the specificity of [99mTc]Tc-pyrophosphate SPECT interpretation by differentiating myocardial tracer uptake from tracer persistent in the cardiac blood pool (19).
(A) Positive [99mTc]Tc-pyrophosphate scan showing Perugini grade 3 uptake with heart–to–contralateral-lung ratio of 2.13 on planar imaging. (B) Diffuse tracer uptake on SPECT images. Contemporary diagnostic standard for [99mTc]Tc-pyrophosphate is SPECT imaging. Planar imaging performed alone leads to high percentage of false-positive studies.
The protocol recommendations for [99mTc]Tc-pyrophosphate scintigraphy are available in an American Society of Nuclear Cardiology practice points document, which was released in 2016 and updated in 2019 (20).
OTHER IMAGING MODALITIES FOR TRANSTHYRETIN CA
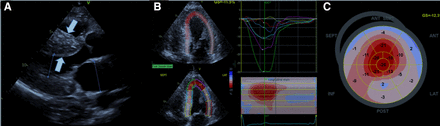
The suspicion of CA most often stems from a finding of unexplained left ventricular hypertrophy on 2-dimensional echocardiography (Fig. 2A). The presence of pericardial effusion (usually small) and an interatrial septum of increased thickness (usually <8 mm) with left ventricular hypertrophy should prompt consideration of infiltrative cardiomyopathy (21). Biatrial enlargement is also commonly seen. The finding of a unique apical-sparing global longitudinal strain abnormality was proposed as a highly sensitive and specific finding in CA (Figs. 2B and 2C) (22). More recently, this finding was reported to have a lower specificity than originally thought (23).
Two-dimensional echocardiography: (A) Parasternal long axis view with left ventricular hypertrophy (white arrows). (B and C) Apical sparing longitudinal strain abnormality.
Cardiac MRI may show a unique abnormality of gadolinium kinetics in CA (nulling to the myocardium before or at the same inversion time as the blood pool). In addition, diffuse subendocardial late gadolinium enhancement (resulting in a train-track appearance when present on the left ventricular and right ventricular sides of the interventricular septum) (Fig. 3) and a very high extent of extracellular volume expansion are characteristic findings. Echocardiography and cardiac MRI do not distinguish between transthyretin and light-chain CA (24,25). Newer radionuclide imaging approaches using 18F-labeled PET tracers are evolving but are not yet in routine clinical use.
Four-chamber view of cardiac MRI showing diffuse subendocardial late gadolinium enhancement. Arrows point to late gadolinium enhancement on left ventricular and right ventricular sides of interventricular septum, resulting in train-track appearance.
Comparative data on the various imaging modalities for transthyretin CA are lacking. Anecdotal evidence indicates that the pathobiology imaged by each modality is distinct. For example, the apical-sparing longitudinal strain abnormality pattern seen on 2-dimensional echocardiography may not parallel the distribution of late gadolinium enhancement on cardiac MRI. Similarly, there is often a discrepancy between the extent of extracellular volume expansion on cardiac MRI and the intensity and distribution of [99mTc]Tc-pyrophosphate in the myocardium. Ongoing comparative studies will almost definitely offer unique insights into the pathobiology of transthyretin CA and, hopefully, provide answers to fundamental questions such as the target of [99mTc]Tc-pyrophosphate binding, which remains a mystery.
POTENTIAL PITFALLS IN THE DIAGNOSIS OF TRANSTHYRETIN CA
Although cardiac scintigraphy with bone-seeking tracers has been truly transformative for the field CA, vigilance for the many potential diagnostic pitfalls is of the utmost importance.
First, the most important step in the evaluation of a patient with suspected CA is the exclusion of light-chain amyloidosis with serum and urine studies for a clonal dyscrasia. The importance of performing these studies on every patient undergoing [99mTc]Tc-pyrophosphate imaging cannot be overstated, unless the clinical context has already obviated exclusion of light-chain amyloidosis. This may be the case when, for example, evaluating carriers of transthyretin amyloidosis gene mutations. When the serum and urine studies are equivocal, a hematology consultation and, often, a tissue biopsy are required (26).
Second, SPECT imaging must always be performed to localize the tracer uptake to the myocardium. Interpretation of [99mTc]Tc-pyrophosphate findings should not be based on planar imaging.
Third, whenever there is a discrepancy between the clinical probability and imaging findings, there must be a low threshold to perform an endomyocardial biopsy. This is particularly true when the clinical findings are suggestive of CA but the serum or urine studies have excluded light-chain CA and the [99mTc]Tc-pyrophosphate images are negative for transthyretin CA. In such cases, TTR gene sequencing should be performed to identify rare variants (e.g., Phe84Leu and Ser97Tyr) that are known to be associated with a negative [99mTc]Tc-pyrophosphate result (27).
The performance characteristics of [99mTc]Tc-pyrophosphate and [99mTc]Tc-DPD have largely been evaluated in expert centers and in patients with a high pretest likelihood of transthyretin CA. With the increasing awareness of CA, these tests are being applied in the community and in patients with a lower pretest probability of disease. During this period of transition, we must remain vigilant for false-positive and false-negative test results.
SUMMARY
The noninvasive diagnosis of cardiac transthyretin amyloidosis using scintigraphy with 99mTc-labeled bone-seeking tracers has been transformative for the field. This ability to make a noninvasive diagnosis without an endomyocardial biopsy has unmasked a previously unrecognized community prevalence of the disease. The new availability of life-prolonging therapy for transthyretin amyloidosis has enhanced the impact of cardiac imaging. Although cardiac scintigraphy for transthyretin amyloidosis has exceptional diagnostic accuracy, careful attention to protocol and interpretation, as well as cognizance of potential pitfalls, is essential to avoid consequential misdiagnoses.
DISCLOSURE
Prem Soman has received grant funding to the institution from Pfizer and is on the consultancy/advisory boards of Pfizer, Alnylam, Spectrum Dynamics, and Eidos. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- Received for publication March 9, 2023.
- Revision received April 10, 2023.