Abstract
Radionuclide therapy with 223Ra-dichloride can be helpful for patients with osteoblastic osseous metastatic disease in the setting of castration-resistant prostate cancer without visceral metastases. This article reviews the indications, proper use and handling, patient work-up before therapy, and many of the technical considerations, including a discussion of coding and billing, along with pitfalls that have been identified.
Early after its discovery in the early 1900s, 223Ra was known as actinium X or radio-actinium (1–6). Much has been learned about this radionuclide since that time. 223Ra-dichloride (Xofigo; Bayer HealthCare), a bone-seeking calcium mimetic, works as an α-particle therapy that binds to areas of increased bony turnover in osseous metastases within the hydroxyapatite matrix, such as 99mTc-methlyene diphosphonate, 99mTc-hydroxydiphosphonate, or 18F-sodium fluoride. Once localized, the α-particle deposits its energy in a highly localized manner, within a range of 100 μm (7), or on the order of a few cells apart from the site of disease. The α-particles deposit high levels of energy that cause predominantly double-stranded DNA breaks in the treatment region, leading to highly localized cell death (8,9) and sparing adjacent normal tissues (10,11). More than a century after the discovery of 223Ra, it was studied to treat osseous metastases in the setting of prostate cancer and found to demonstrate a survival benefit in the landmark Alpharadin in Symptomatic Prostate Cancer Patients (ALSYMPCA) study (12). It was subsequently approved by the Food and Drug Administration on May 15, 2013 (13), for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases, and no known visceral metastatic disease (14). ALSYMPCA demonstrated not only a survival benefit but also favorable effects on patient quality of life and a favorable safety profile (12).
After lung cancer, prostate cancer is the most common cancer in men in the United States. It affects 1 in 8 men and is the cause of death in 1 in 41 men (15). Osseous metastatic disease in the setting of prostate cancer occurs in 70%–90% of patients (16,17). Prostate cancer patients with osseous metastatic disease are known to have a lower quality of life, an increased cost of care, and higher mortality (17,18). Therefore, radium therapy can have a clear impact on these patients. The ALSYMPCA trial demonstrated a survival benefit of 14.9 mo in the treated group versus 11.3 mo in the untreated group, which received the best standard of care plus a placebo (12). Though this seems a short time, it does amount to a 30% longer time frame than in patients who did not receive 223Ra-dichloride.
PATIENT SELECTION, CLINICAL CONSIDERATIONS, AND PROTOCOL
The Food and Drug Administration approved use of 223Ra-dichloride for treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases, and no known visceral metastatic disease. Castration-resistant cancer is cancer that continues to grow even when the testosterone levels are at or below the castrate level; it is also known as hormone-refractory or hormone-resistant prostate cancer (19). Careful screening of prior imaging for visceral metastatic disease in organs and lymph nodes is helpful; the package insert advises an upper limit of 3 cm for lymph nodes (14). Clinical guidelines indicate that patients should undergo CT of the chest, abdomen, and pelvis (if not already performed) before therapy to assess for visceral metastatic disease (20). Prostate bed disease or localized involvement of the urinary bladder is not considered visceral disease (20). Hematologic analysis must also be performed if the patient does not have visceral metastases and is being considered for therapy. Per the package insert, before the first treatment the absolute neutrophil count should be at least 1.5 × 109/L, the platelet count at least 100 × 109/L, and the hemoglobin at least 10 g/dL (14). Blood work should be done within at least 30 d of the initial therapy (20). There is some expected impact on bone marrow, and the patient will need follow-up blood count analysis before additional therapies. For subsequent administration of the radionuclide therapy, the patient should have an absolute neutrophil count of 1 × 109/L and a platelet count of 50 × 109/L; no hemoglobin limit is required for subsequent therapies (Table 1) (14).
Clinical Eligibility Criteria for Therapy (14)
Because this therapy localizes to regions of bony turnover, it is imperative that a patient undergo bone scanning before being considered for therapy. If the osseous metastases do not take up tracer, the patient will not benefit from this therapy and should not receive it. Pretherapy bone scans can be done with SPECT or PET bone scanning agents. Clinical guidelines indicate that there should be at least 2 osseous metastatic lesions (20). PET agents such as prostate-specific membrane antigen and fluciclovine are not a substitute for bone-seeking tracers in this setting (21).
Increasingly, nuclear medicine professionals are seeing patients in consultation before therapies and are using evaluation and management codes. This change is beneficial for patients, as they are not seen only on the day of therapy but rather have time to prepare for potentially unexpected radiation safety instructions. Consultations also allow patients or caregivers to ask questions and may ease patient concerns about the therapy and its side effects. Nuclear medicine professionals also benefit by having time to review the patient’s history; coordinate with the urologist, oncologist, or other clinical colleagues who referred the patient; and obtain insurance preauthorization. During the consultation process, needed items can be identified, such as a bone scan or blood work, which can then be obtained in a timelier manner so as not to delay care or result in a wasted therapy dose.
Understanding prior therapies the patient has had is also important. 223Ra-dichloride when given in conjunction with abiraterone therapy + prednisone or prednisolone has been associated with an increased frequency of fractures, compared with patients who did not receive the 223Ra-dichloride (22). This association has caused the European Medicines Agency to recommend a contraindication in this setting. This recommendation is not reflected in the Food and Drug Administration’s package insert at this time, but nonetheless, current guidelines discourage this practice (20). Other myelosuppressive therapies the patient may have had in the 4 wk before therapy may also set the patient up for more profound myelosuppression. Likewise, if patients have had external-beam hemibody radiation or other systemic radionuclides within 24 wk of therapy, careful consideration of the risk-to-benefit ratio of therapy is recommended (20). If patients have epidural tumor or spinal cord compression, they should be treated preferentially with external-beam radiation therapy before 223Ra-dichloride (20). Working together with clinical colleagues to settle on an appropriate time for 223Ra-dichloride therapy is important.
Assessing performance status is recommended. This assessment can be done with the Eastern Cooperative Oncology Group or Zubrod performance status criteria (23). Guidelines suggest that life expectancy should be at least greater than 6 mo, and an Eastern Cooperative Oncology Group performance status of 0 to 2 is preferred (20). Guidelines also recommend documentation of pain and patient-related symptoms before and during therapy to evaluate the patient’s quality of life (20,22).
Because of the complexity of tracking the many elements of therapy, it can be helpful to use a checklist for each patient. Example items to keep on such a checklist are in Table 2.
Items to Include in 223Ra-Dichloride Therapy Checklist and Consultation Note
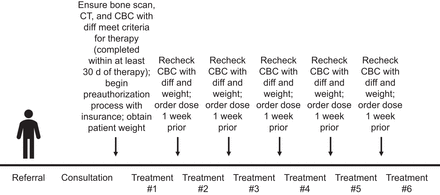
Provided the patient meets the laboratory parameters and functional status requirements, therapy is given intravenously every 4 wk for a total of 6 therapies. If there is evidence of myelosuppression after a therapy, the next therapy can be delayed for to up to 6–8 wk. If blood counts do not improve despite supportive care, no further treatment is given (14). Table 3 reviews important clinical metrics to evaluate before therapy. Figure 1 illustrates the therapy procedure at a glance.
Elements to Review Before Treatment on Day of Therapy
Therapy process. CBC = complete blood count; diff = differential.
RADIOPHARMACEUTICAL THERAPY PROPERTIES AND DOSIMETRY
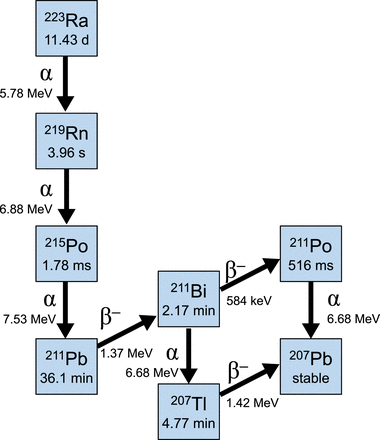
223Ra is an α-particle and has a half-life of 11.43 d. Most of the therapy (95.6% abundance) is α-particles, which deposit high energy with a very short pathlength. β-particles make up 3.6% of the decay, and γ-photons make up 1.1% (14). These γ-photons allow for easy measurement on standard dose calibrators and survey meters. The full decay schema is described in Figure 2, and the dosimetry is detailed in Table 4 (24). Given the dosimetric data, one can understand why gastrointestinal symptoms may occur, as the radiopharmaceutical is excreted predominantly via the gastrointestinal tract in healthy individuals.
Decay schema for 223Ra-dichloride.
223Ra-Dichloride Dosimetry (24)
RADIATION SAFETY PRECAUTIONS, PATIENT CONSENT, AND AS-NEEDED MEDICATIONS
According to the Nuclear Regulatory Commission, all radionuclide therapies should be given under the auspices of a quality management program (20). A written directive is required for each 223Ra-dichloride therapy for each individual (i.e., 6 written directives for an individual patient for the entire course of therapy). Written directives include the administered activity, which in this case is weight-based (55.13 kBq [1.49 μCi]/kg), and an updated weight will be necessary for ordering doses a week before therapy. Once again, assessment of the complete blood count with differential will be necessary to determine whether the patient is experiencing myelosuppression and needs a pause or discontinuation of therapy.
During the clinical consultation, there should be careful discussion of the radiation safety precautions, side effects, and complications. When discussing precautions to take after the therapy, nuclear medicine professionals should primarily focus on stool and body fluids. Patients should be told to sit while urinating to minimize splatter and that careful handwashing after using the restroom is imperative. If there is a urinary catheter, patients should be told to use disposable gloves when manipulating or changing it. Instructions are also recommended on disposal of any materials that could contain body products. As part of consenting to the therapy, the patient should understand the potential side effects. The most common are myelosuppression, diarrhea, nausea, and peripheral edema. As-needed medications for nausea and diarrhea may be prescribed, or over-the-counter versions can be recommended. Patients should also be made aware of the potential for a transient increase in bone pain, often referred to as the flare phenomenon (20). Because of this potential, and because a patient may already have pain at baseline, the clinician who treats that pain should be told of the possible need for an increase in medication, so it can be arranged for in advance.
Complications that have been reported from ALSYMPCA are myelosuppression (13%), neutropenia (2%), thrombocytopenia (7%), and grade 5 treatment-emergent adverse events (13%). Long-term follow-up of patients from the trial yielded no suggestion of myelodysplastic syndrome, acute myelogenous leukemia, or new primary bone cancer. Secondary non–treatment-related malignancies occurred in 4 patients treated with 223Ra-dichloride, whereas 3 occurred in placebo patients. One patient experienced aplastic anemia 16 mo after the last injection (12).
DAY-OF-THERAPY TECHNICAL CONSIDERATIONS
Although external radiation exposure from 223Ra-dichloride is quite low compared with other therapies, care in administration is key. Care should be taken to avoid internal radiation contamination through injection, inhalation, or skin absorption. Because α-particles deposit their energy in a highly localized range, greater damage can occur than with β-particles or γ-photons. Because of the long half-life, spills should be avoided but can be identified with standard survey equipment because of the γ-photons. Contamination can be cleaned up with a dilute aqueous ethylenediaminetetraacetic acid solution, and areas can be resurveyed to ensure that they are decontaminated.
Before administration, the therapeutic dose should be measured, absorbent shielding should be placed under the patient’s arm to prevent spillage, and the intravenous catheter should be checked for patency. The therapy is often given with a 3-way stopcock with a saline flush to ensure maximal delivery; all intravenous lines and connections should be secure. Because this is an α-particle therapy, more localized damage may occur in the event of extravasation. A shield is recommended to keep the dose to the individual delivering the therapy as low as reasonably achievable. Dose rates are surprisingly high near the point source itself (Table 5). Some institutions choose to use a test dose of a small amount of 99mTc-pertechnetate and acquire dynamic images to ensure a patent intravenous catheter. A slow intravenous injection over 1 min is used. After injection, flushing with isotonic saline is recommended (20).
Dose Rates Comparing 99mTc Vs. 223Ra Derived from Exposure Rate Constants (μSv/h per MBq) (31)
CREATING A SUCCESSFUL RADIONUCLIDE THERAPY PROGRAM WITH 223RA-DICHLORIDE
According to the manufacturer, Bayer HealthCare, technical prerequisites for site initiation include obtaining a radioactive materials license approved for medical use of 223Ra-dichloride, creating an authorized-user list for medical application of 223Ra-dichloride, providing documentation regarding training on handling and use of 223Ra-dichloride, and verifying the accuracy of dose calibrators to measure 223Ra-dichloride activity. Dose calibrator testing with a National Institute of Standards and Technology–traceable 223Ra standard is provided by Cardinal Health. Additional prerequisites, which may already be established in a nuclear medicine area, include monitoring occupational doses and securing areas for storage, waste disposal, and inventory management (25).
Some studies have established data that 223Ra-dichloride is cost-competitive as compared with other prostate cancer therapies. In addition, 223Ra-dichloride therapy has been found to result in fewer skeleton-related events than other therapies, and other groups have demonstrated a slower decline of quality of life over time, with meaningful improvement, in patients who receive this therapy than in those who receive placebo (12,26).
After a site has been initiated, understanding the codes involved in billing is important (Table 6). If the therapy is administered in a hospital setting, working closely with the hospital billing office is important, as is staying attuned to potential annual changes in Centers for Medicare and Medicaid Services coding practices. Both inside and outside the hospital, Xofigo Access Services offers guidance on coding (25). Before therapy is administered, it is recommended that insurance companies be queried on the need for prior authorization. The time needed for prior authorization is quite variable among different insurance providers, but 15 or more business days may be needed. Failure to use codes can result in denial. In addition, failure to allow enough time for preauthorization or for managing clinician and patient expectations can also be problematic. To ensure that patients do not have an out-of-pocket burden, close work with institutional billing offices or Xofigo Access Services should allow them to provide guidance on issues encountered in coding and billing. In addition, the Society of Nuclear Medicine and Molecular Imaging website has a “Coding and Reimbursement Q&A” section with resources available to members (27).
Current Codes in 223Ra-Dichloride Therapy (27)
In addition to these items, it is imperative to provide patients with multidisciplinary care and identify clinicians of various backgrounds who will be involved in therapy referral and follow-up. Follow-up practices may differ on the basis of institutional protocols. Some practices include mid-therapy evaluation with laboratory biomarkers or imaging; however, no well-defined guidelines exist yet.
LOOKING AHEAD
The use of 223Ra-dichloride is likely to evolve, considering the recent Food and Drug Administration approval of 177Lu-vipvotide tetraxetan (also known as 177Lu-PSMA-617) (28). 223Ra-dichloride is a bone-based therapy, whereas 177Lu-vipvotide tetraxetan is a therapy that can also target soft-tissue lesions. Holistic evaluation and the tracer uptake profile of the patient may be needed to assess which agent would be of most benefit.
Another area of interest may be repeat 223Ra-dichloride therapy. Some research has already been done in this area, and the findings suggest that the therapy is well tolerated and provides additional control of osseous metastatic disease (29,30). Longer-term follow-up may be helpful to evaluate for continued long-term safety.
EDUCATION AND RECOMMENDED READING
There is a good deal of helpful information available to those starting a 223Ra-dichloride therapy program. Some of the higher-yield materials include the 223Ra practice parameters of the American College of Radiology, American College of Nuclear Medicine, American Society for Radiation Oncology, and Society of Nuclear Medicine and Molecular Imaging (20); the ALSYMPCA trial (12); and Xofigo access services (25).
CONCLUSION
223Ra-dichloride remains an effective therapy that improves survival for patients with osseous metastatic disease related to castration-resistant prostate cancer in the absence of visceral metastatic disease.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. Complete the test online no later than September 2025. Your online test will be scored immediately. You may make 3 attempts to pass the test and must answer 75% of the questions correctly to receive Continuing Education Hour (CEH) credit. Credit amounts can be found in the SNMMI Learning Center Activity. SNMMI members will have their CEH credit added to their VOICE transcript automatically; nonmembers will be able to print out a CE certificate upon successfully completing the test. The online test is free to SNMMI members; nonmembers must pay $15.00 by credit card when logging onto the website to take the test.
Published online Jul. 26, 2022.
REFERENCES
- Received for publication April 1, 2022.
- Accepted for publication July 13, 2022.