Abstract
The 2018 Food and Drug Administration approval of 177Lu-DOTATATE for the treatment of somatostatin receptor–positive neuroendocrine tumors (NETs) represents a paradigm-shifting approach to cancer treatments around the globe. Gastroenteropancreatic NETs overexpress the somatostatin subtype receptor 2, which is now exploited for receptor-based imaging and therapy, thus generating significant progress in the diagnosis and treatment of this orphan disease. The recent Food and Drug Administration approval of receptor-based PET radiopharmaceuticals and a new peptide receptor radiopharmaceutical therapy, 177Lu-DOTATATE, has dramatically impacted NET patient management. The focus of this paper is to review clinical considerations associated with implementing a 177Lu-DOTATATE program. We review receptor-based NET radiopharmaceuticals; 177Lu-DOTATATE patient selection criteria; administration methods; clinical, regulatory, and radiation safety considerations; technical factors; tissue dosimetry; and reimbursement guidelines.
It is estimated that the annual incidence of neuroendocrine tumors (NETs) is 7 per 100,000 persons, resulting in approximately 23,000 new cases each year in the United States (1). NETs are slightly more common in women (52.7%), with 5-y overall survival depending strongly on the grade and stage of disease. At the time of diagnosis, approximately half of patients presents with localized disease whereas the other half has already progressed to regional disease or distant metastases. Localized disease is often well managed by surgery alone (2), with median overall survivals in the range of 4–30 y depending on site and grade (1).
NETs of gastroenteropancreatic origin often secrete serotonin and a variety of other peptide hormones that can cause characteristic symptoms known as carcinoid syndrome or other symptoms related to the tumor’s site of origin. Shortly after the discovery of somatostatin receptors (SSTRs) in 1972 (3), it was observed that agents targeted to subtype 2 of the somatostatin receptor resulted in potent antisecretory effects in NETs, providing significant palliative benefit in patients with secreting gastroenteropancreatic NETs. These SSTR-targeted somatostatin analog (SSA) agents were initially available in short-acting immediate-release formulations (octreotide acetate, 1988) and were later made available in long-acting formulations (octreotide and lanreotide, 1998–2001) (4). In addition to the palliative therapeutic benefit of SSAs, these agents were found to exhibit antitumor effects (5,6), resulting in their clinical use as primary interventions for metastatic gastroenteropancreatic NETs regardless of hormone secretion status.
Expression of SSTR is observed in many cancer types, and this receptor is highly overexpressed in low-grade (grades 1 or 2) NETs (mitotic rate ≤ 20, Ki-67 index ≤ 20%) and to a lesser extent in high-grade (grade 3) NETs (mitotic rate > 20, Ki-67 index > 20%) (7,8). Based on the high degree of overexpression, as well as the known molecular structures with high-affinity binding to this receptor, research into the use of radiolabeled SSAs for imaging and therapy began in the early 1990s. The first proof-of-concept nuclear imaging studies used [123I-Tyr3]-octreotide (9,10); shortly thereafter, use of 111In-pentetreotide (an octreotide analog labeled with diethylenetriaminepentaacetic acid chelator for complexation of 111In) gained traction for NET imaging, receiving Food and Drug Administration approval in 1994 (11,12). In subsequent years, SSAs with macrocycle chelators (DOTATOC, DOTANOC, DOTATATE) were developed and shown to have improved stability, biodistribution, and clearance for a variety of radiometal labels (13,14). Among these, 68Ga-DOTATATE, 68Ga-DOTATOC, and 64Cu-DOTATATE have thus far received Food and Drug Administration approval and are in current clinical use, whereas 111In-pentetreotide is being phased out in favor of the newer PET-based imaging agents.
Early success in the diagnostic imaging of gastroenteropancreatic NETs led to the development of SSTR-targeted radiotherapeutics, often referred to as peptide receptor radionuclide therapy (PRRT) in this context. The first therapeutic agent to be studied was 90Y-DOTATOC, which was shown to have significant oncologic benefit in small animals and humans (13,15,16). Trials with 90Y-DOTATOC demonstrated some renal and hematologic toxicity, and these off-target effects were found to be dose-limiting for this agent. Most recently, 177Lu (half-life [t½], 6.6 d)-labeled DOTATATE has been favored because of increased retention time in tumors, an apparent reduction in nephrotoxicity, as well as logistical considerations associated with these agents. In the phase 3 NETTER-1 trial, 177Lu-DOTATATE was evaluated in patients with well-differentiated, unresectable or metastatic, progressive midgut NETs (17). In comparison to long-acting octreotide, 177Lu-DOTATATE was associated with an improved response rate (18% vs. 3%, P < 0.001) and progression-free survival (65.2% vs. 10.8% at 20 mo). These data led to the Food and Drug Administration approval of 177Lu-DOTATATE (Lutathera; Advanced Accelerator Applications USA Inc.) in 2018 for treatment of patients with SSTR-positive gastroenteropancreatic NETs. This therapeutic agent is now widely available and frequently used in the treatment of patients with NETs. As of 2021, Advanced Accelerator Applications reported that 177Lu-DOTATATE is available at more than 230 treatment centers in the United States.
The purpose of this paper is to review the practical clinical considerations associated with 177Lu-DOTATATE, with an emphasis on what the care team members (technologists, nurses, pharmacists, physicists) need to know for successful application of this newly approved PPRT agent.
PATIENT SELECTION
177Lu-DOTATATE is not currently considered a first-line therapy for NET. Instead, patients with surgically unresectable, metastatic, or locally advanced midgut NET may be treated with first-line SSA therapy. If disease progresses during SSA therapy and SSTR positivity is confirmed with functional imaging, the patient may be considered for 177Lu-DOTATATE therapy (18). A multidisciplinary team (nuclear medicine, medical oncology, endocrinology, surgical oncology, interventional radiology, radiation oncology) should evaluate the patient’s performance status, clinical and imaging data, potential alternative treatments, and PRRT contraindications before deciding to proceed with PRRT. Adequate bone, liver, and renal function should be verified with the European Neuroendocrine Tumor Society exclusion criteria detailed in Table 1, and the patient’s Karnofsky performance, a measure of health status, should be at least 60% (19).
PRRT Exclusion Criteria Considerations (19)
Fundamental to patient selection is the clinical behavior of the gastroenteropancreatic NET, often determined by the tumor’s primary site, grade, and differentiation. NET grade reflects the proliferative activity of cells, measured by mitotic rate or Ki-67 index, and differentiation describes the extent to which tumor cells resemble their healthy endogenous cell line (20). Gastroenteropancreatic neuroendocrine neoplasms were recently subdivided in the 2019 World Health Organization classification system (21) and are summarized in Table 2. NET tumor grade inversely correlates with SSTR density and prognosis; in general, the lower the grade, the higher the SSTR density. A high SSTR density thus correlates with an improved response to PRRT and better prognosis (20). Because of weak or absent SSTR expression, as well as being generally more aggressive, higher-grade NETs and poorly differentiated neuroendocrine cancers have a worse prognosis (22). SSTR positivity for all lesions should be confirmed with SSTR imaging before 177Lu-DOTATATE therapy; PET-based SSTR imaging (DOTATATE, DOTATOC, DOTANOC) has become the standard of care and is preferred over 111In-pentetreotide scintigraphy because of the higher spatial resolution and dramatically improved lesion detectability of these agents (23). Lesions with uptake more intense than normal liver activity are deemed SSTR-positive and thus better candidates for PRRT (24).
Classification and Grading Criteria for Neuroendocrine Neoplasms of Gastrointestinal Tract and Hepatopancreatobiliary Organs (21)
Because of the frequent lack of SSTR expression in higher grade (grades 2 or 3) and poorly differentiated NETs, these tumors are often examined with 18F-FDG PET imaging in lieu of, or in addition to, SSTR PET imaging. PRRT administration has historically been contraindicated in patients with sites of discordant or mismatched lesions (lesions with positive 18F-FDG uptake, positive contrast enhancement on CT or MRI, and negative SSTR expression). 18F-FDG–positive lesions are known to be associated with a reduced likelihood of response to PRRT. In this patient population, multidisciplinary teams may consider the addition of concomitant chemotherapy to a PRRT regimen. Higher-grade NETs (grades 2 or 3) are currently being evaluated in the phase III NETTER-2 trial, which is investigating PRRT as first-line therapy when used in combination with long-acting, high-dose octreotide (22). The phase III COMPETE trial is currently comparing 4 cycles of 7.4 GBq (200 mCi) of 177Lu-DOTATOC with daily everolimus administration in patients with SSTR-positive disease (25). Further studies of 177Lu-DOTATATE are being conducted on pediatric patient populations, including the NETTER-P study, as well as investigator-initiated studies (26).
CLINICAL CONSIDERATIONS
Patient preparation is a component critical to the success of PRRT. Many NET patients receive SSAs for symptomatic control of their disease, and the SSAs elicit their therapeutic effect by binding to SSTRs. 177Lu-DOTATATE also works by targeting SSTRs, and administration of SSAs should be carefully planned during 177Lu-DOTATATE treatment to prevent receptor saturation, which can interfere with the efficacy of PRRT (24,27). Long-acting SSAs should be discontinued at least 4 wk before each 177Lu-DOTATATE dose; short-acting SSAs may be used as needed up to 24 h before each treatment. SSAs may be resumed 4 h after administration of 177Lu-DOTATATE for symptomatic management between therapeutic cycles and after completion of treatment (24).
During 177Lu-DOTATATE administration, patients should be monitored for potential reactions to the infusion (24,27). Although infrequent, extravasation of the radiopharmaceutical may occur if the intravenous line becomes obstructed; patency of the line should be verified before the start of the infusion and monitored throughout the administration. Signs of extravasation, such as pain and swelling, should be immediately addressed to increase the clearance of the radiopharmaceutical from the infusion site. Steps to be taken include image acquisition to confirm and quantify the amount of extravasated radiopharmaceutical (whole-body planar scintigraphy and SPECT/CT of the affected area), elevation and exercise of the affected arm as much as possible for 24 h, and application of a compression bandage with heated gel pads for 20 min every 6 h to facilitate redistribution of the radiopharmaceutical. After the initial 24 h, imaging should be repeated. A qualified medical physicist should be consulted regarding the radiation dosimetry of this event, and referral to plastic surgery should be considered on the basis of the dosimetry results. Additional information can be found in literature case reports and reviews (28,29).
In addition to the risk of extravasation, patients may experience a neuroendocrine hormonal crisis during 177Lu-DOTATATE administration due to excessive hormone released by the tumor (30). Symptoms include cutaneous flushing, diarrhea, bronchospasm, and hypotension and generally occur during or within 24 h of the initial 177Lu-DOTATATE dose. A hormonal crisis can be treated by intravenous administration of SSAs and fluids, corticosteroids, and correction of electrolyte imbalances (24,30). 177Lu-DOTATATE may be administered in an outpatient setting not immediately equipped to deal with a carcinoid crisis. Institutional policies describing how to obtain additional medical support or transport the patient to an emergency clinic, if needed, should be in place.
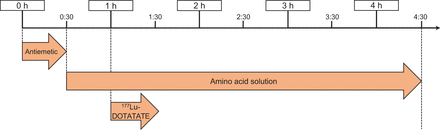
Since the kidneys receive a significant radiation dose, amino acids should be given simultaneously with each cycle of 177Lu-DOTATATE to decrease absorption through the proximal tubules, thus reducing the radiation dose to the kidneys (27,31,32). The amino acid solution must be infused over 4 h and should contain 18–25 g each of l-lysine HCl and l-arginine HCl in a total volume of 1–2 L. Several commercial amino acid solutions are available that contain the required amounts of lysine and arginine. However, the presence of additional amino acids in these products may cause significant nausea and vomiting for the patient. Alternatively, a 2-amino-acid solution containing only lysine and arginine may be compounded by the hospital or local pharmacy to improve patient tolerability (33,34). Figure 1 illustrates the time line for administration of 177Lu-DOTATATE PRRT. Antiemetics are administered first, followed by the start of the amino acid infusion 30 min later. The amino acid infusion should run at a rate that allows for the entire volume to be infused over a total of 4 h. Administration of 177Lu-DOTATATE begins 30 min after the start of the amino acid infusion. If the 177Lu-DOTATATE prescribed activity is decreased, the amount of amino acids administered is not altered (24).
177Lu-DOTATATE PRRT administration time line.
Long-term radiation effects of 177Lu-DOTATATE treatment may occur and can include myelosuppression and renal toxicity (24,27,31). Laboratory values, including complete blood count and renal function tests, should be monitored throughout the treatment cycle and after the completion of PRRT to assess for toxicity. On the basis of acute changes, typically myelosuppression, the prescribed 177Lu-DOTATATE activity can be reduced, withheld, or permanently discontinued (24).
The use of 177Lu-DOTATATE in specific populations may require additional clinical considerations. Pregnancy status should be verified in patients with reproductive potential before initiation of therapy, as 177Lu-DOTATATE can harm the fetus. All patients should be counseled on the use of effective contraception during and after treatment and advised of the potential for infertility. Patients who are lactating should be advised not to breastfeed during the treatment cycle and for 2.5 mo after the conclusion of therapy.
Dose adjustment is not automatically necessary for mild to moderate renal impairment; however, renal function should be monitored more frequently in these patients. Decreased renal function can lead to a longer residence time in the kidneys and higher exposure rates and may require dose adjustment for subsequent cycles. Limited data are available on the safety of 177Lu-DOTATATE in patients with severe renal impairment or end-stage renal disease (35), but it is not a contraindication for treatment.
Caution should be exercised when considering PRRT in patients with extensive peritoneal carcinomatosis because of the risk of radiation-induced bowel obstruction. Spontaneous urinary incontinence may make the safe administration of PRRT impossible. Additional PRRT clinical considerations can be found in consensus guidelines by Hicks et al. (19). Because special situations can vary in complexity, the multidisciplinary team should communicate and tailor the treatment plan to each patient’s individual needs.
REGULATORY AND RADIATION SAFETY CONSIDERATIONS
Before initiating a PRRT program, sites will need to ensure that their radioactive materials license includes the possession and use of 177Lu in sufficient quantities to cover ordered doses as well as residual waste material (36). It is also important to review the waste disposal policy with the site radiation safety officer. Although the t½ of 177Lu is relatively short (6.6 d), 177Lu-DOTATATE may contain small amounts (∼0.1%) of the long-lived contaminant 177mLu (t½, 161 d). This contaminant can make it difficult to comply with the waste storage and disposal requirement (<120-d t½) outlined in Nuclear Regulatory Commission title 10 of Code of Federal Regulations, part 35, section 35.92. If decay in storage is not a viable option, the radiation safety officer or nuclear pharmacist can coordinate pickup and disposal with the local radiopharmacy or a third-party vendor (36,37).
The Nuclear Regulatory Commission requires the authorized user physician to date and sign a directive containing the patient’s name, radiopharmaceutical, prescribed administered activity, and route of administration before the 177Lu-DOTATATE is administered. The nuclear medicine staff should follow the site’s procedure for administration of therapeutic radiopharmaceuticals, including verifying patient identity, verifying activity to be administered, and administering the drug per the written directive (36).
Care should be taken when handling and administering 177Lu-DOTATATE to keep radiation exposure as low as reasonably achievable for the staff and general public (37). Appropriate personal protective equipment should be worn, and shielding and tongs should be used for manipulation of the 177Lu-DOTATATE vial. 177Lu-DOTATATE is shipped by Advanced Accelerator Applications or Novartis directly to the end-user site as a 7.4-GBq (200 mCi) quantity in a shielded vial. A variety of methods have been developed for direct infusion from the vial, or the activity may be drawn up into a shielded syringe for use in a syringe pump (24). For patients requiring a reduced 3.7-GBq (100 mCi) administration, the site can use an infusion pump to administer the correct volume of 177Lu-DOTATATE from the unit dose vial. Alternatively, the vial can be adjusted by aseptically withdrawing the excess volume of 177Lu-DOTATATE using a shielded syringe; the residual volume of 177Lu-DOTATATE should be properly disposed of according to the site’s waste disposal policy.
Except in the case of medical events, only the patient and nuclear medicine staff should be in the treatment room from the start of the infusion until the patient has been released. The patient should also have access to a single-user restroom during the visit because urine will be radioactive after infusion. If a medical event does occur, all steps should be taken to ensure the medical safety of the patient without regard for personnel radiation exposure. After the medical intervention, the qualified medical physicist or radiation safety officer may provide radiation dose estimates for unbadged personnel who participated in patient care. The patient may be released after therapy, provided the radiation dose to the maximally exposed member of the public is expected to be less than 5 mSv (0.5 rem). The patient must be provided with written instructions on how to follow as-low-as-reasonably-achievable principles, including interruption or discontinuation of breastfeeding, if applicable; restroom use; interaction with household family members; and other considerations deemed relevant by the authorized user or radiation safety officer (38). 177Lu-DOTATATE is usually administered as an outpatient procedure, as exposure to the general public after the infusion is unlikely to exceed Nuclear Regulatory Commission limits (39).
PROTOCOL AND TECHNICAL CONSIDERATIONS
After a patient has been approved for treatment, the product is ordered through the manufacturer’s Web-based ordering system. The manufacturer confirms via email, unless the desired treatment date is within 3 d, in which case the ordering facility must call the manufacturer to verify material availability. The manufacturer recommends that subsequent treatments be scheduled when the first treatment is scheduled. After production, the manufacturer ships the radiopharmaceutical to the end user under quarantine; the 177Lu-DOTATATE cannot be infused into the patient until the batch-release document from the manufacturer is received via email, thus releasing the lot from quarantine. The radiopharmaceutical is supplied in a 30-mL unit dose vial containing 7.4 GBq (200 mCi) ± 10% of 177Lu-DOTATATE in 20.5–25 mL at a concentration of 370 MBq/mL (10 mCi/mL) calibrated to the time of infusion. The default 177Lu dose calibrator setting may be used for measuring 177Lu-DOTATATE, but it is recommended that end users obtain an annual calibration source from Advanced Accelerator Applications or Novartis to determine a more precise dial setting. Currently, approved labeling for 177Lu-DOTATATE describes only a 3.7 or 7.4 GBq (100 or 200 mCi) administration, and there appears to be minimal dose calibrator geometry effect (40,41) for volume modification between these levels.
Several different radiopharmaceutical administration methods have been reported (27,42). The gravity method arose from the NETTER-1 clinical trial experience. This method involves hanging a normal saline bag and connecting it via an intravenous line to an upright shielded 177Lu-DOTATATE vial with the needle tip above the level of the contents. A longer needle is inserted into and touches the bottom of the 177Lu DOTATATE vial, which is connected to the patient administration intravenous line. The saline entering the closed system at the top of the vial pushes the radiopharmaceutical out through the elongated needle placed at the bottom of the vial and into the patient. Further reading regarding the gravity infusion method is included in Hope et al. (27) and the manufacturer’s package insert (24). There have been issues reported with this technique (43), including loss of pressure in the vial to the room air from improper needle placement through the vial septum. Because U.S. Pharmacopeia general chapter <825> radiopharmaceutical guidelines were published in February 2021 (44) and have already been adopted in some areas, it is imperative to follow these guidelines for beyond-use times after puncturing a vial septum to ensure patient safety.
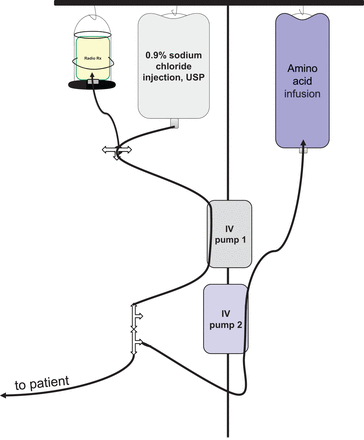
Our institution uses a 177Lu-DOTATATE secondary pump infusion method (45) similar to the Rotterdam method (Fig. 2). For this method, the 177Lu-DOTATATE vial is placed into a shield, spiked with a vial spike administration set, inverted, and infused with an intravenous pump. Using this simple and easily reproducible method, we have observed very little residual activity in the administration vial, essentially no contamination, and marginal additional exposure to the technical staff (46). Alternatively, the activity may be drawn up into a shielded syringe for administration via a syringe pump (47). Regardless of the 177Lu-DOTATATE administration method used, the timing of antiemetics and amino acid infusion before and immediately after the 177Lu-DOTATATE administration should not be modified with any alternative administration technique.
177Lu-DOTATATE Rotterdam secondary pump infusion method. IV = intravenous; Radio Rx = 177Lu-DOTATATE; USP = U.S. Pharmacopeia.
DOSIMETRY AND IMAGING
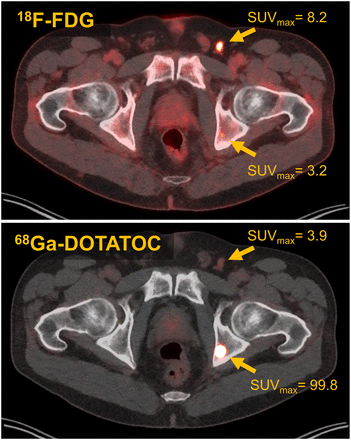
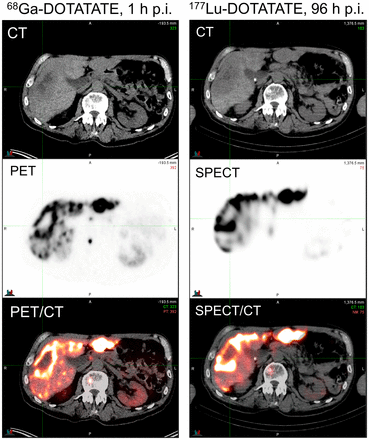
Imaging plays a major role in the management of patients with NETs. During workup, patients undergo a PET/CT study (68Ga-DOTATATE, 68Ga-DOTATOC, or 64Cu-DOTATATE) to assess for SSTR receptor positivity. Patients with low uptake of the SSTR-targeted radiotracer, in comparison to the liver or spleen, should not be considered eligible for treatment with 177Lu-DOTATATE. In some cases, it can be helpful to also obtain a 18F-FDG PET/CT study to identify lesions with increased metabolic rates. Determining lesion 18F-FDG positivity, as well as discordant tracer uptake (18F-FDG–positive and DOTATATE/TOC–negative), provides prognostic value beyond what standard histopathologic grading can provide (48). An example of 18F-FDG and 68Ga-DOTATOC discordance is shown in Figure 3. Although tumor uptake on pretreatment SSTR PET imaging weakly correlates with absorbed dose from 177Lu-DOTATATE therapy and likelihood of response, it is not possible to accurately predict absorbed dose to tumors and normal organs from pretreatment PET imaging. This impossibility is due to the short t½ of 68Ga (68 min), typically conducive to imaging at about 1 h after radiopharmaceutical administration. Tumor uptake of DOTATATE/TOC typically peaks several hours after administration, and the clearance kinetics must be characterized for accurate absorbed dose determination. The t½ of 64Cu (12.7 h) may be sufficient to obtain quantitative information at later PET imaging time points (2–3 d) (49), but this sufficiency has yet to be demonstrated conclusively in the literature. For these reasons, it is most common to perform dosimetry by SPECT/CT or planar γ-imaging after administration of the therapeutic quantity of 177Lu-DOTATATE. An example of DOTATATE imaging, both pretreatment PET/CT and posttreatment SPECT/CT, from our own institution is shown in Figure 4. In addition to providing quantitative information for dosimetry, posttreatment imaging is useful for rapid evaluation of whether any extravasation has occurred during infusion of the 177Lu-DOTATATE. Although rare, extravasation can require immediate medical intervention to prevent excess radiation exposure at the site of injection.
Example of discordant uptake on 18F-FDG and 68Ga-DOTATOC imaging. Two lesions are visualized: left inguinal node (18F-FDG–positive, minimal 68Ga-DOTATOC uptake) and left ischium bone lesion (68Ga-DOTATOC–positive, 18F-FDG–negative).
PET/CT imaging 1 h after injection of 68Ga-DOTATATE, and SPECT/CT imaging 96 h after injection of 177Lu-DOTATATE. p.i. = after injection.
177Lu emits 2 photons that can be used for imaging: 113 keV in 6.2% of decays and 208 keV in 10.4% of decays. Details regarding quantitative SPECT imaging of 177Lu can be found in MIRD pamphlet no. 26 (50). Typical acquisition parameters include the use of a medium-energy collimator, an auto-contouring orbit, at least 60 views per head, 15–30 s/view, a 128 × 128 or higher matrix size, a 15%–20% energy window on the 208-keV photopeak, and 5%–10% scatter windows above and below the 208-keV photopeak. Images are typically reconstructed using iterative techniques with CT-based attenuation correction, triple-energy-window scatter correction, collimator detector response modeling, iterative updates adequate to achieve activity recovery convergence (e.g., 12i8s for typical 3-dimensional ordered-subsets expectation maximization), and minimal or no pre- or postreconstruction filtering. In addition to these acquisition and reconstruction parameters, system sensitivity should be assessed via an appropriate phantom experiment (51), and dead time should be estimated from measured patient count rates during imaging (52).
Fully calibrated and corrected images can then be used to assess patient-specific dosimetry. Methods for determination of patient-specific absorbed dose vary in complexity and accuracy; there are an increasing number of software tools to facilitate dose calculation from radiopharmaceuticals (53). The interested reader can find pertinent dosimetry details in the papers by Graves et al., Bolch et al., and Siegel et al., as well as from the MIRD Primer for Absorbed Dose Calculations, Revised (published in 1991, new edition expected in early 2022) (54–57). Tissues of relevance in dosimetry calculations for 177Lu-DOTATATE often include bone marrow, kidneys, and occasionally liver in cases of prior or planned liver-directed therapy or extensive hepatic tumor burden. Details of normal-tissue dose limits for radiopharmaceuticals can be found in the recent article by Wahl et al. (58).
BILLING AND CODING
On January 1, 2019, the Centers for Medicare and Medicaid Services issued a Healthcare Common Procedure Coding System code of A9513 to 177Lu-DOTATATE. The A9513 code descriptor specifies billing as per 1 mCi, and it is important to ensure that the administered millicurie amount for the therapy is accurately documented and submitted (59). If a portion of the 177Lu-DOTATATE activity is wasted because of personalized dosimetry or other reasons, a JW modifier should be used. The JW modifier is used to report discarded drug amounts still eligible for payment under Medicare’s discarded drug policy (60). The Healthcare Common Procedure Coding System codes for antiemetics and amino acids will vary with physician drug choice and amino acid procurement location. Current Procedural Terminology codes are also used for 177Lu-DOTATATE treatment. Current Procedural Terminology code 79101 (radiopharmaceutical therapy, intravenous administration) can be used for 177Lu-DOTATATE administration. The first hour of intravenous amino acid administration can be coded with 96365, and subsequent hours can be coded with 96366. Coding for antiemetic premedication will vary with drug type and route of administration (59).
Medical billing and coding guidelines can vary by practice and region, and readers are encouraged to consult the SNMMI’s coding and reimbursement Web pages, manufacturer’s reimbursement guide, and internal institutional reimbursement specialists. The accurate coding and classification of a patient’s diagnosis and treatment are essential, and billing modifiers may be required. Billing codes and reimbursement rates are subject to change based on payer, date of service, and billing setting, and the information shared at the time of this publication is no guarantee of reimbursement. Billing and coding guidelines will continue to evolve with the growth of PRRT. Additionally, reimbursement approaches to dosimetry-guided radiopharmaceutical therapy are emerging, as detailed by Graves et al. (61).
CONCLUSION
177Lu-DOTATATE currently serves as a second-line treatment option for patients with surgically unresectable, metastatic, or locally advanced midgut NETs that have failed first-line SSA therapy and is a paradigm-shifting approach to cancer treatment. 177Lu-DOTATATE is paving the way to the future of receptor-based therapy and personalized cancer treatment; this PRRT agent has yielded significant treatment progress for NETs and dramatically impacted patient management. Before offering a patient 177Lu-DOTATATE therapy, a multidisciplinary team should evaluate patient-specific clinical considerations, and SSTR positivity should be confirmed with functional imaging. Sites wanting to implement a 177Lu-DOTATATE program are encouraged to consider the patient selection criteria; PRRT administration methods; clinical, regulatory, and radiation safety considerations; technical factors; tissue dosimetry; and reimbursement practices required with this newly approved PRRT agent.
DISCLOSURE
Nic Mastascusa has served as a consultant for Novartis Inc., and Stephen Graves has a research proposal being reviewed by Novartis Inc. relating to 177Lu-DOTATATE. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank our respective families and colleagues for their ongoing support.
Footnotes
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. Complete the test online no later than September 2025. Your online test will be scored immediately. You may make 3 attempts to pass the test and must answer 75% of the questions correctly to receive Continuing Education Hour (CEH) credit. Credit amounts can be found in the SNMMI Learning Center Activity. SNMMI members will have their CEH credit added to their VOICE transcript automatically; nonmembers will be able to print out a CE certificate upon successfully completing the test. The online test is free to SNMMI members; nonmembers must pay $15.00 by credit card when logging onto the website to take the test.
Published online Jun. 14, 2022.
REFERENCES
- Received for publication January 10, 2022.
- Accepted for publication June 8, 2022.