Abstract
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. Complete the test online no later than March 2025. Your online test will be scored immediately. You may make 3 attempts to pass the test and must answer 75% of the questions correctly to receive Continuing Education Hour (CEH) credit. Credit amounts can be found in the SNMMI Learning Center Activity. SNMMI members will have their CEH credit added to their VOICE transcript automatically; nonmembers will be able to print out a CE certificate upon successfully completing the test. The online test is free to SNMMI members; nonmembers must pay $15.00 by credit card when logging onto the website to take the test.
The emergence of PET and MRI as a hybrid modality has demanded new approaches to protocols and procedures. Although protocols for MRI and PET individually lend themselves to synergistic and simultaneous approaches, there are a number of unique challenges and patient preparations that require consideration. This article provides insight into the protocols, procedures, and challenges associated with simultaneous PET/MRI in both adult and pediatric populations. Although protocols may be specific to applications or pathologies of interest, a richer discussion of the clinical applications of PET/MRI is beyond the scope of this article and will be detailed in part 4 of the series. The foundation of PET/MRI protocols is an understanding of the various MRI sequences, which are outlined succinctly. The principles outlined for protocols and procedures are general, and specific application will vary among departments. Given that the procedures for PET are well established among the readership of this journal, this article emphasizes MRI factors unless specific variations in standard PET protocols or procedures are driven by the simultaneous MRI. This article is the third in a 4-part integrated series sponsored by the PET/MR and Publication Committees of the Society of Nuclear Medicine and Molecular Imaging–Technologist Section.
The emergence of PET fused with CT required variations in protocols to accommodate the sequential PET/CT procedure. The more complex and time-consuming protocols and procedures for MRI simultaneously obtained with PET require more careful consideration. Although most PET/MRI investigations use 18F-FDG, several other 18F tracers and those chelated to radiometals, such as 68Ga and 64Cu, are expected to emerge. There are 3 main aspects of PET/MRI protocols and procedures: patient preparation for both MRI and PET procedures, the imaging procedures themselves, and quality assurance procedures. Patient preparation and the imaging protocol need to be considered for both adult and pediatric populations.
PET/MRI combines the high sensitivity and quantification of molecular-level tracers of PET with the exquisite soft-tissue contrast and some functional imaging parameters of MRI (1). Despite these benefits, the major limitation is the complex array of MRI sequences that could be performed and the time cost per bed position. For PET/CT, 2–4 min per bed position is typical of a whole-body scan, but in clinical PET/MRI, as many as 5 MRI sequences could require 5–10 min per bed position (1). There are several benefits to optimizing the MRI sequence timing to the standardized PET bed position. First, it maintains the consistency associated with quantitation (e.g., SUV) between and within sites. Second, extending PET bed position timing has a marginally increased impact (particularly during the latter part of the acquisition) on both radionuclide decay and target-to-background ratio. Recent developments in ultrafast MRI sequences have shortened the MRI acquisition to 3–5 min per bed position (1), although 2–4 min might be considered optimal. It is possible to adjust protocols for MRI sequences beyond the PET bed position timing to accommodate the extended MRI sequences after the PET acquisition is completed or, alternatively, 15 min before commencing the PET acquisition.
MRI SEQUENCES
A typical MRI examination consists of imaging the body part of interest multiple times with varying parameters that produce images with different tissue weightings. Each tissue weighting serves to provide a different clinical insight. PET/MRI is generally a whole-body examination, which for most represents imaging from the base of the skull to the thighs, consistent with approaches to PET/CT. The absence of the CT radiation dose issues for the brain and eyes means that it has become more common in PET/MRI to image the vertex through the thighs. For some indications or in a pediatric setting, the vertex of the skull to the toes is the norm. PET/MRI acquires the PET and MRI data simultaneously, with each bed position having 2–4 different MR image sequences. Conversely, PET/CT is acquired sequentially with a rapid CT component. Thus, if the MRI sequences selected do not prolong the bed position, PET/MRI could be a faster acquisition by the margin of the CT acquisition (seconds). Nonetheless, PET/MRI also has sequential MRI acquisitions with the localizer before the PET acquisition and the potential for additional sequences without PET at the end that extend the protocol marginally (minutes) but are generally negligible in the overall protocol. Both the contrast protocols and the positioning of the patient confound these times. Nonetheless, optimizing the MRI sequences for PET/MRI is critical for patient comfort and compliance and for the diagnostic integrity of the examination. Perhaps the simplest way to consider sequences is to first consider T1- and T2-weighted images and then explore specific sequences. The following sequences are not exhaustive but represent the more common sequences in MRI (the more detailed discussion will be reserved for those sequences relevant specifically to PET/MRI protocols): spin echo (T1 short repetition time and echo time, T2 long repetition time and echo time, fast spin echo, dual echo, and proton density–weighted long repetition time and short echo time); gradient echo (GRE) (susceptibility-weighted imaging; T1-weighted volume-interpolated spoiled GRE, which could be referred to as volume-interpolated breath-hold examination [VIBE] or liver acquisition with volume acquisition, depending on the manufacturer; T2 and T2*; steady-state free precession; and dual GRE); inversion recovery (short-tau inversion recovery [STIR] fat suppression and fluid-attenuated [long tau] inversion recovery [FLAIR] fluid suppression); T1- or T2-weighted imaging; diffusion-weighted imaging (DWI) (apparent diffusion coefficient maps [postprocessed sequence]); diffusion tensor imaging and tractography of nerves; perfusion-weighted imaging (T1 gadolinium contrast-enhanced and arterial spin labeling [noncontrast technique]); functional MRI; MR angiography (MRA) (contrast-enhanced MRA, time-of-flight angiography [without or with contrast], and phase-contrast MRI [noncontrast technique]). More detailed treatment of less frequent, less relevant (to PET/MRI), or more novel sequences is beyond the scope of this article and can be explored in the broader literature.
In PET images, the shade of gray or color represents count density or counts per pixel or per voxel. In CT, the shade of gray represents the degree of attenuation or tissue density (2). For MRI, the shade of gray of tissues represents signal intensity, with white typically being a high signal intensity and black being a low signal intensity. In PET, relative quantitation is undertaken visually or with calculations that compare the count density on the structure of interest to a reference tissue. Examples include a tumor-to-liver comparison and a comparison of the regional cerebral cortex to the contralateral side or cerebellum. For MRI, intensity is also compared between the tissue of interest and reference tissues, with the term hyperintense indicating whiter, brighter, greater intensity than the reference tissue; hypointense indicating darker or lower intensity than the reference tissue; and isointense meaning the same brightness or intensity as the reference tissue (e.g., a brain tumor relative to surrounding brain tissue).
The image formation process and pulse sequences have been explained in a previous article in this series (3), but there is a need to briefly define several specific terms. After radiofrequency excitation, the time for the signal to return to equilibrium is called relaxation time and the signal produced is referred to as free induction decay (4,5). The time between each radiofrequency pulse is called the repetition time (4,5). The echo signal is termed spin echo, is stronger than the free induction decay signal, and is measured at the peak time of the echo (4,5). Free induction decay is formed by a single radiofrequency pulse (often thought of as 90° but technically not necessarily). GRE is formed by 1 radiofrequency pulse with a gradient reversal. Spin echo is formed with 2 radiofrequency pulses (e.g., 90° and 180°), and stimulated echo is formed with 3 or more radiofrequency pulses. Hahn echo is a term generally used to refer to echo produced by radiofrequency pulses other than 90° and 180° (but technically could include 90° and 180° pulses) (4,5).
T1 Images
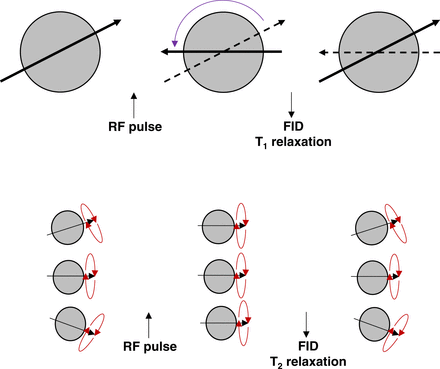
T1-weighted images are a standard part of any MRI protocol. As the term suggests, the T1 pulse sequence highlights differences between tissues based on T1 relaxation times or longitudinal relaxation (sometimes referred to as spin lattice relaxation time) (3–6). As outlined in Figure 1, the proton dipoles align with the magnetic field after the radiofrequency pulse. The proton dipoles then revert back (relax) to nearly their original orientation. The time it takes an individual tissue to relax results in different signal intensities. For example, water content, air, and bone have a slow relaxation, which produces a lower signal and a darker representation on images. Conversely, fat, protein-rich fluid, and slow-flowing blood have a rapid relaxation time that produces a whiter or brighter signal on images. T1-weighted spin echo produces the truest T1 signal but generally takes longer to acquire, typically anywhere from 2 to 5 min. An alternative option is to use T1-weighted GRE, which uses the properties of the gradient coils inside the MR scanner to generate the T1-weighted images faster than conventional spin echo techniques. Clearly, this is advantageous for simultaneous PET/MRI, for which bed position time needs to be optimized, but as outlined in the previous article in this series (3), the coils themselves can produce artifacts in the PET images. GRE images are also more susceptible to artifacts caused by inhomogeneity in the magnetic field as a result of metal or blood products (e.g., pixel swap). Pixel swap is an artifact associated with the Dixon technique, in which the algorithm confuses water and fat pixels; this artifact is most typical of the extremities or of areas with a low fat signal. These sequences are also more susceptible to motion artifacts and anatomic wraparound artifacts. Regardless, T1-weighted GRE allows multiple sequences with imaging for less than 30 s. These phenomena can be further exploited using paramagnetic contrast agents (e.g., gadolinium) to produce several variations to the T1 pulse sequence.
Schematic representation of T1 signal produced by longitudinal relaxation. Proton dipoles have net magnetism in magnetic field (left) but become aligned after radiofrequency pulse (middle) and produce T1 signal on relaxation (right). B0 = magnetic field; M = magnetism; RF = radiofrequency.
Gadolinium Enhancement and Fat Suppression
T1 signal intensity is amplified by gadolinium-based contrast agents, which, compared with noncontrast T1 images, produce brighter (more intense) signals from tissues. In effect, this brightness appears to shorten the T1 relaxation times, given that the intensity of slow-relaxation-time tissues has now become brighter. Although this phenomenon helps us understand the process, the reality is that the relaxation time is not actually shortened; instead, the signal itself is increased from tissues. One application would be in diseased tissue in which increased perfusion or tissue permeability (e.g., injury, tumor, or inflammation) results in a higher concentration of contrast agent and a disproportionate increase in signal intensity compared with normal tissues.
Unfortunately, fat tissues are already very intense, and increases in intensity may not be easily distinguished from normally intense tissues. As a result, fat suppression sequences are typically imaged after contrast administration to reduce the fat signal (e.g., phase-contrast techniques and inversion recovery sequences). These sequences might also be referred to as fat-attenuated or fat saturation. Nonetheless, both fat-saturated and non–fat-saturated images provide useful insights, but in the context of multiple sequences and time cost, it is not convenient to run both pulse sequences. An alternative is the Dixon dual-echo sequence, which uses algorithms and the inherent properties of the signal (chemical shift) generated by fat and water in MRI to generate both fat-saturated and non–fat-saturated image sets from a single acquisition. In essence, water and fat precess at different rates, which means cyclically they will be in-phase and out-of-phase (a little like the seconds hand of a clock being out-of-phase with the minutes hand until each minute that they approximately become in-phase). By acquiring in-phase and out-of-phase images, the algorithm can produce 4 separate sequences: in-phase (water plus fat), out-of-phase (water minus fat), fat (in-phase minus out-of-phase), and water- or fat-suppressed/fat-saturated (in-phase plus out-of-phase). Acquisition of an ultra-fast GRE Dixon technique requires about 15–20 s per bed position. In addition, this acquisition is 3-dimensional and can be reformatted into multiple planes during postprocessing with minimal image resolution loss. This technique is essential for generating the MR attenuation correction sequence maps for the PET data (3).
T2 Images
As with T1-weighted images, T2 pulse sequences are a standard part of most MRI protocols and might also be referred to as T2-weighted images. As these terms suggest, the T2 pulse sequence highlights differences between tissues based on T2 relaxation times or transverse relaxation (sometimes referred to as spin–spin relaxation) (4,5). As outlined in Figure 2, the proton dipoles align with the magnetic field after the radiofrequency pulse. The proton dipoles then revert back (relax) to nearly their original orientation for the T1 signal. Each proton dipole also has precession altered in alignment by the radiofrequency pulse. The time it takes an individual tissue to relax precession alignment results in different signal intensities. For example, water content and brain gray matter produce a high intensity signal whereas muscle, fat, and white brain matter produce intermediate intensities. A minor issue relating to T2 sequences is the influence of inhomogeneous magnetic fields on tissue T2 relaxation times, sometimes referred to as T2*. T2 then is the truest T2 signal using a spin-echo sequence. Gadolinium contrast medium is not used because it shortens T2 relaxation and thus suppresses rather than amplifies the signal; T2 sequences are therefore run before contrast enhancement. The exception is the T2 FLAIR steady-state GRE sequence, which shows contrast enhancement due to a mixed T1 and T2 signal.
Schematic representation of difference between T1 (top) and T2 (bottom) signal production. For T1, proton dipoles become aligned after radiofrequency pulse and produce T1 signal on relaxation. T2 signals relate to precession of proton dipoles relaxing back to ground state. FID = free induction decay; RF = radiofrequency.
Typically, T2-weighted images are used to visualize a pathologic process such as edema. Again, there are several ways to achieve this goal. Traditional spin echo techniques to obtain T2 images are time-prohibitive, and thus, several methodologies have been developed to decrease the time it takes to acquire them. The fastest of these is ultra-fast spin echo, which has image acquisition times under a minute typically. Another option is the fast relaxation fast spin echo technique; this technique is slower than the ultra-fast spin echo technique but substantially faster than spin echo. Since both fat and water produce a bright signal on T2 imaging, obtaining fat-saturated T2 images can help better define the nature of what is visualized. Like T1 sequences, fat suppression can be achieved with STIR sequences, which are useful from an imaging perspective but limited by the 5-min sequence time.
Proton Density
Proton density sequences use the nature of MRI (proton or hydrogen ion imaging) to image the density of protons (4). Tissues with high density or proton intensity include fluid and fat, similar to T2. Since the technique was useful in differentiating high-intensity fluid from low-intensity fibrocartilage and intermediate-intensity hyaline cartilage, it is often used for joint imaging, with FLAIR displacing its use in brain imaging.
Spin Echo
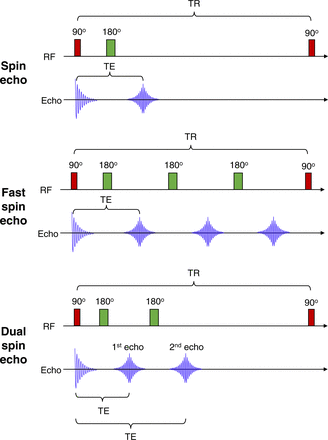
Spin echo uses 90° radiofrequency excitation pulses (Fig. 3) to flip longitudinal magnetism (T1) and dephases transverse magnetism (T2). The subsequent 180° radiofrequency-refocusing pulse rephases the spins to produce coherence (4,5). Thus, recovery of transverse magnetism produces a spin echo. Fast spin echo is the same principle as spin echo (Fig. 3) except it uses a series of rapidly applied 180° radiofrequency-rephasing pulses to produce multiple echoes (echo train length) within the same repetition time (4,5), allowing more rapid data collection but having a limit on echo train length (typically less than 7 for T1). The echo time may vary from echo to echo in the train and may produce different characteristics (contrast vs. resolution, for example). T1 is generated from the initial echoes and T2 from the later echoes. Longer echo train lengths with short echo times degrade contrast and produce blur but may be useful for enhanced T2 images. Dual echo (Fig. 3) is the same principle as fast spin echo with an echo train length of 2, with the first echo usually being proton density and the second T2-weighted imaging.
Schematic representation of MR sequences. Spin echo uses 90° followed by 180° radiofrequency pulse to produce echo. Fast spin echo uses 90° followed by multiple 180° radiofrequency pulse to produce multiple echoes. Dual spin echo, as name suggests, produces 2 echoes from 90° followed by repeated 180° radiofrequency pulse. TE = echo time; TR = repetition time.
GRE
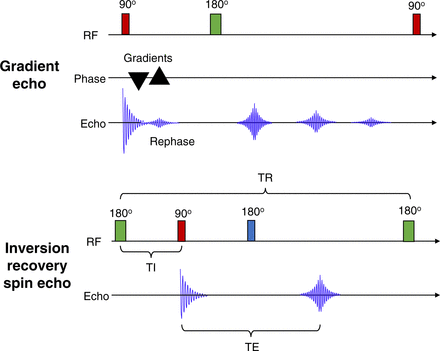
GRE uses bipolar gradient pulses after the 90° excitation radiofrequency pulse (4,5). For standard GRE, there is no 180° refocusing radiofrequency pulse as depicted in Figure 4. The addition of one or more of the 180° refocusing pulses produces fast GRE sequences. Standard GRE uses a negatively pulsed gradient to dephase the spin, which is then rephased with a second positively pulsed gradient, generating the echo signal independently of the 180° refocusing radiofrequency pulse.
(Top) Schematic representation of GRE in which 90° radiofrequency pulse is followed by bipolar gradients first dephasing free induction decay and then rephasing free induction decay. (Bottom) Schematic representation of inversion recovery spin echo in which 180° radiofrequency pulse is followed by 90° radiofrequency pulse. RF = radiofrequency; TE = echo time; TR = repetition time.
Inversion Recovery
STIR is a fat suppression sequence (Fig. 4) that uses an inversion time for T1 in which the inversion time is ln2 (∼0.693) multiplied by the T1 for fat. The sequence works because the T1s for fat and water are different (4,5). In the same way, water suppression can be accomplished using FLAIR sequences (FLAIR can resemble T2 images). Although these approaches produce fairly homogeneous suppression of water or fat, they are not specific for fat and water so can cause decreased demarcation of tissues. Furthermore, they are incompatible with gadolinium contrast administration because the apparent shortening of contrast-enhanced tissues will be impacted (suppressed) by a short inversion recovery time.
DWI
DWI is a type of MRI that shows how fluid is moving through tissues (extracellular space). Using a strong gradient, greater diffusion results in greater dephasing, and the signal is therefore reduced. As a result, DWI is effective in identifying restricted diffusion of water molecules as a higher signal intensity. DWI can be used to assess tissue edema, ischemia, and cellularity (e.g., proliferation of tumor cells). DWI represents an image set with different b-values. The b-value is a value that reflects gradient strength and timing, and so, the higher the b-value the stronger the diffusion. DWI is a combination of true diffusion values and the T2 signal, and therefore, the lower the b-value the more T2-weighted the image is (4). Different tissues and resultant pathologies exhibit diffusion behaviors different from random Brownian motion to constrained motion in 1 voxel direction. Injury that changes water diffusion will change the DWI signal. Diffusion tensor imaging is a variation on DWI that measures diffusion in multiple directions, which allows mapping of nerve fibers and tractography. An apparent diffusion coefficient map is literally a map or tensor of the actual diffusion values for tissues without the influence of T2.
Other Sequences
Functional MRI is a cluster of MRI approaches designed to reveal regional or time-dependent variations in MR signal and is associated primarily with brain imaging. Functional MRI targets a variety of physiologic parameters and uses numerous MRI sequences. Perfusion-weighted imaging is mostly gadolinium T1 images but also arterial spin labeling without gadolinium (4). Susceptibility-weighted imaging sequences exploit the T2* sequence susceptibility to small fluctuations in magnetic field and, consequently, can distinguish calcium from blood. This ability is useful in differentiating blood products in various pathologic processes.
MRA generates images with a high degree of contrast between tissues and blood. The images are associated with blood flow rather than vessel structures. Some aspects of flow are predictable and can be used to generate image contrast, whereas other aspects of flow can create procedure difficulties or artifacts (e.g., turbulence, velocity, or direction changes). There are several approaches to MRA. The first is contrast-enhanced MRA, which uses gadolinium contrast agents to shorten the T1 relaxation time and enhance the brightness of blood signals (4,5). Time-of-flight MRA does not use contrast agents but instead captures the unsaturated spins of flowing blood with GRE sequences to produce a bright vascular image (4,5). Phase-contrast MRA uses amplitude and phase information to image blood flow velocity (4,5).
PATIENT PREPARATION
Patients need to undergo the preparation associated with both the PET scan and the MRI scan. For PET, this preparation will include fasting and instructions regarding radiation safety. For MRI, the screening questionnaire for magnetic safety and gadolinium contrast needs to be completed. Additionally, PET imposes limitations on strenuous activity and requires management of any patients who are diabetic. Patients need to be screened for metals to ensure there are no unsafe implants or devices going into the MRI environment. A lot of the prescreening can be accomplished as the exam is being ordered or scheduled by asking just a few questions targeting implants that could contraindicate the exam (e.g., pacemakers, magnetic spinal rods, cochlear implants, or palate expanders) or that could impact image quality (e.g., dental spacers or braces). When patients arrive for their appointment, they are also required to fill out a more comprehensive MRI metal screening form, which is then reviewed verbally with trained MRI personnel to ensure safety. Patients should also undergo a visual inspection before entering zone 4 (the PET/MRI scan room) as a final verification that nothing unsafe is going into the magnet.
Patient history can be especially useful in image interpretation, and taking a robust patient history is therefore an essential part of patient preparation. In oncology, for example, some patients receive bone marrow–stimulating medications at the end of a cycle of therapy that may alter biodistribution of the radiopharmaceutical in PET imaging. If a delay in scheduling after these peak effects is not ideal, this information will assist with the accurate interpretation of the PET/MRI data.
Protocols vary across clinical sites and across radiopharmaceuticals; however, an uptake phase of 45–90 min is typical. Patients are usually injected intravenously in an ambient environment to minimize stimulation. Recent investigations have demonstrated higher dose extravasation rates associated with manual injection using a syringe/needle or syringe/butterfly apparatus (7–9). Lower extravasation rates are achieved with canula use with autoinjector infusion. For patients undergoing gadolinium contrast sequences, a single canula might be used for both the PET tracer administration and the later gadolinium administration. Clearly, care with line security is crucial throughout the uptake phase and patient positioning.
Patient compliance is an important consideration given the extended time of the PET/MRI procedure. Compared with MRI, PET has the additional requirements of fasting and blood sugar level adjustment, in addition to the long uptake phase without stimulation. Compared with PET, MRI has the additional time and complexity associated with coil and patient setup, screening for magnetism-susceptible objects, and noise. Additionally, the PET/MRI gantry can create issues associated with claustrophobia, even in patients with no previous history of claustrophobia. For some patients, compliance requires the preadministration of anxiolytics such as diazepam. In some cases, sedation or general anesthesia may be required, adding complexity to both the protocol and patient care. Sedation and general anesthesia are generally not initiated until at least 30 min after administration of the PET radiopharmaceutical but in some circumstances may be administered immediately after radiopharmaceutical injection.
PET/MRI PROTOCOLS
PET acquisition parameters are generally the same for both PET/CT and PET/MRI. Each bed position is acquired at 2- to 4-min intervals. In PET/CT, the PET portion determines the bulk of the length of the procedure, with CT being performed very quickly in a sequential model. Contrast CT protocols clearly extend the overall imaging procedure. Conversely, in PET/MRI, the images are acquired simultaneously and PET cannot progress to the next bed position until all the MRI sequences are completed. PET/MRI procedures commence with a localizer, comparable to the topogram in a PET/CT scan, to be used for planning both the PET bed positions and the MRI sequences at each bed position. In PET/MRI, a specific MR attenuation correction sequence is obtained at each bed position to create the attenuation correction maps for PET reconstruction. In addition, depending on the region being imaged and the pathology of interest, several other sequences will be performed at each bed position. Although the Dixon method is referenced in this article for attenuation correction, there are several other approaches to attenuation correction in clinical practice, as well as others emerging from development as detailed in the second article in this series (3).
Reducing artifacts from respiratory motion is an important consideration. Breath-hold techniques can be effective if the sequence is short enough and the patient is compliant. Such techniques work particularly well for the short-duration T1-weighted images, but even ultrafast sequences are too long for T2 breath-hold approaches. Respiratory triggering can be used, or images can be gated, or synchronized, to the patient’s respiratory cycle. Respiratory gating works best when patients are breathing at a steady regular rate. The third option performs respiratory motion correction based on liver motion during respiration by tracking an MRI voxel at the apex of the liver.
More recent interest in accelerated protocols with 4-, 3-, 2-, and even 1-min bed positions have spurred debate about the benefits of reduced time and the image quality for PET and MRI (1). The counter debate is that compromising a full MRI sequence to minimize the time per bed position undermines the value and insights of MRI. Nonetheless, a hybrid protocol might permit a longer application of a suite of MRI sequences and a longer PET acquisition (or potentially dynamic imaging during the uptake phase) for the single bed position of interest, followed by reduced sequences and standard PET bed positions for the remainder of the acquisition.
MRI-only acquisitions during the radiopharmaceutical uptake time may assist in reducing the acquisition time associated with each PET/MRI bed position. For example, MR images of the total spine that are acquired before the combined PET/MRI sequence can be coregistered with the latter if the patient has not moved, with gadolinium contrast spine sequences added after the PET/MRI sequence. This approach could save the patient considerable time and optimize the PET/MRI sequence, especially when considering that the duration of precontrast total-spine imaging can vary between 30 and 90 min depending on patient-related factors.
Whole-Body Oncology PET/MRI
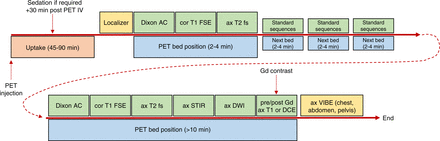
Although protocols will vary substantially from site to site and depending on equipment and clinical indications, for the purpose of a general overview it is useful to consolidate whole-body PET/MRI protocols into several scenarios. In each scenario, the MRI sequence commences with a localizer scan followed by attenuation correction and then by T1, T2, and then special sequences if appropriate. The first approach (1,10) is a fairly standard set of 5 MRI sequences per bed position with a total acquisition time of 5–8 min per bed position that includes T1-weighted Dixon for attenuation (<15 s), DWI with 3 b-values (almost 1.5 min), T1-weighted volume-interpolated spoiled GRE (VIBE) (<30 s), T2-weighted single-shot half spin echo (∼30 s to 1 min), T2-weighted STIR (2 min), and T1-weighted volume-interpolated spoiled GRE, after contrast administration if appropriate (18 s).
In this first approach to PET/MRI, after the conclusion of the whole-body exam a more specific region may be added to the standard sequences, for example, the prostate bed. For this approach, it would be prudent to perform the whole-body PET/MRI first to capitalize on disease localization, enhance PET target-to-background contrast in the targeted region, and acquire critical data to avoid noncompliance issues.
To minimize MRI sequence timing to better match each PET bed position without compromising diagnostic quality, some axial MR images can be replaced with images in the coronal plane. Typically, 3 axial bed positions are equivalent to 1 bed position in the coronal plane. For example, high-quality T2 and STIR sequences can be run in the coronal plane. Additionally, a coronal VIBE can be imaged in addition to the axial plane VIBE.
A second approach uses a wider range of sequences per bed position for the whole-body acquisition, and these might vary depending on the nature of the investigation. The result is a more comprehensive suite of MRI sequences for the whole-body PET/MRI but a longer scanning time beyond 10 min for key bed positions corresponding to the region of interest (Fig. 5). In this scenario, a basic set of sequences (e.g., 3) might be applied to each bed position whereas the broader suite is applied to the key bed position (including long PET imaging time). The basic sequences might be Dixon attenuation correction, coronal T1 fast spin echo (or coronal VIBE to include fat saturation), and axial T2-weighted imaging, for example. Advanced sequences are applied to specific bed positions covering regions of specific interest and may include, without being limited to, T2 fast spin echo, STIR, DWI, dynamic contrast-enhanced, and pre- and postcontrast T1. Specifically, PET/MRI with a focus on liver neoplasia might insert the extended suite of sequences as bed position 3 of 5 and include a breath-hold technique. The thoracic region might insert the extended suite over 2 bed positions and include respiratory gating. Head and neck cancer would have the longer sequences at bed position 1, and pelvic pathology would also have the broader suite of sequences at bed position one, because for most whole-body applications imaging commences from the head and progresses to the thighs. For the pelvis, given bladder excretion of PET tracers, imaging starts at the pelvis and progresses to the head.
Flowchart of example of PET/MRI sequence used for whole-body oncology studies. 3D = 3-dimensional; AC = attenuation correction; ax = axial; cor = coronal; DCE = dynamic contrast-enhanced; DSC = dynamic susceptibility contrast; fs = fat saturation; FSE = fast spin echo; Gd = gadolinium; IV = intravenous injection; LGE = late gadolinium enhancement; SPAIR = spectral attenuated inversion recovery; T1WI = T1-weighted imaging.
This second approach has the advantage of a single imaging session with all data included in a single whole-body protocol. Unfortunately, this approach can increase the time per bed position and, thus, impact the SUV for each bed position because the uptake time after injection for each bed position is progressively extended. Longer sequences could threaten later bed positions if compliance becomes an issue. Importantly, an increased MRI sequence time increases the potential for heating of patient tissues and implants, posing a safety issue. In both approaches, the use of dedicated imaging increases the quality of the procedure by combining the attributes of PET with the MRI sequences. Further, the PET/MRI combination enriches, deepens, and broadens anatomic, physiologic, and biochemical insights regarding tissues and organs.
Neurologic and Cardiac PET/MRI
The value of PET/MRI in the brain and heart arises from the simultaneous acquisition and coregistration of structural information provided by standard MRI sequences, physiologic insights provided by advanced MRI sequences, and molecular and metabolic status provided by PET (11). Although rapid PET protocols have emerged for brain and cardiac imaging in 5 min for a single bed position, 10–15 min for a single bed position are more typical. This longer window (compared with whole-body oncology bed positions) allows a broader range of MRI sequences without extending the length of the study. It is also possible that a standard PET/MRI study of the brain or heart with 10–15 min per bed position might be followed by a whole-body PET/MRI with 3–5 min per bed position. In this scenario, the sequences are different from the sequence for the whole body, reflecting both the change in time per bed position and the purpose of the MRI examination.
PET/MRI of the brain generally requires the following sequences (Fig. 6): attenuation correction with T1-weighted Dixon GRE; conventional brain MRI with T1-weighted, T2-weighted, diffusion-weighted, susceptibility-weighted, and contrast-enhanced T1-weighted imaging; advanced sequences with perfusion-weighted imaging, functional MRI, diffusion tensor imaging, contrast-enhanced T1-weighted imaging, fast GRE, MR spectroscopy, and FLAIR, depending on the clinical indication; and simultaneous PET acquisition with any of several radiotracers, including 18F-FDG, 3′-deoxy-3′-18F-fluorothymidine, 18F-fluoromisonidazole, 18F-florbetapir, O-(2-18F-fluoroethyl)-l-tyrosine, or 6-18F-fluoro-l-dopa.
Flowchart of example of PET/MRI sequence used for brain studies (top) and cardiac studies (bottom). 3D = 3-dimensional; AC = attenuation correction; ax = axial; DCE = dynamic contrast-enhanced; DSC = dynamic susceptibility contrast; FSE = fast spin echo; Gd = gadolinium; IV = intravenous injection; LGE = late gadolinium enhancement; SPAIR = spectral attenuated inversion recovery; T1WI = T1-weighted imaging.
PET/MRI of the heart generally requires the following sequences (Fig. 6): attenuation correction with T1-weighted Dixon GRE; conventional cardiac MRI with T1 fast spin echo, T2 spectral attenuated inversion recovery, and contrast-enhanced T1-weighted imaging; advanced sequences with late gadolinium enhancement, rest perfusion (GRE, echoplanar imaging, and steady state free precession) and wall motion (electrocardiography gating, harmonic phase analysis, and spatial modulation of magnetization), depending on clinical indication; and simultaneous PET acquisition with any of several radiotracers, including 18F-FDG for viability, perfusion tracers based on 82Rb, 13N, or 18F, and novel inflammatory or amyloid markers.
CONCLUSION
PET/MRI is a relatively new imaging modality that, to establish a reliable niche in the imaging market, requires development of universal, practical, and reliable protocols. Protocol development should have a foundation of evidence-based standards for PET, for MRI, and for PET/MRI combined. Patient compliance and diagnostic integrity are central factors for protocol development. Although the complexity of PET/MRI protocols appears onerous, sequence rationalization has produced universally accepted streamlined protocols that fit within the time constraints of standard PET bed positions.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online September 28, 2021.
REFERENCES
- Received for publication May 6, 2021.
- Revision received August 20, 2021.