Abstract
18F-fluciclovine is a Food and Drug Administration–approved PET tracer indicated for patients suspected to have recurrent prostate cancer based on a prostate-specific antigen rise after prior therapy. 18F-fluciclovine PET/CT is performed significantly differently from 18F-FDG PET/CT and requires special attention to patient preparation, injection technique, and imaging time. This article aims to provide nuclear medicine technologists with the best-practice guidelines for the 18F-fluciclovine PET/CT protocol.
Fluciclovine labeled with 18F (18F-fluciclovine) (Axumin; Blue Earth Diagnostics, Inc.) is an amino acid analog PET radiotracer that was approved in 2016 by the U.S. Food and Drug Administration (FDA) for imaging of patients with suspected recurrent prostate cancer based on an elevated level of prostate-specific antigen (PSA) after therapy.
In prior clinical trials, 18F-fluciclovine PET/CT demonstrated higher diagnostic performance than conventional imaging modalities in the localization of recurrent prostate cancer, along with higher specificity in detection of small nodal disease (1–3). When compared with 11C- or 18F-choline, 18F-fluciclovine had similar to slightly higher diagnostic performance in recurrent prostate cancer (4). Preliminary data on the newly investigated prostate-specific membrane antigen ligand PET tracers in recurrent prostate cancer showed higher diagnostic performance than for 18F-fluciclovine (5). More conclusive prospective clinical studies comparing prostate-specific membrane antigen with 18F-fluciclovine are soon to be published. For therapy planning in recurrent prostate cancer, 18F-fluciclovine PET/CT detected additional findings leading to major changes in management (6,7).
Historically, 18F-FDG was the only FDA-approved and the most clinically used PET radiotracer for cancer imaging. Although having clinical experience with 18F-FDG PET/CT might provide a technologist with overall knowledge on the standard operating procedure for PET imaging, 18F-fluciclovine is a new PET radiotracer with a different imaging protocol (Table 1). Thus, it is important that all staff involved in 18F-fluciclovine image acquisition obtain adequate training to ensure consistency and a high level of image quality.
Differences Between Imaging Protocols for 18F-Fluciclovine PET/CT and 18F-FDG PET/CT
Since the FDA approval of 18F-fluciclovine for clinical use, our institution has imaged over 240 patients, ranking us as one of the largest providers of 18F-fluciclovine PET/CT services in the U.S. Midwest. Also, having board-certified nuclear medicine physicians with an average of 10 y of combined expertise in research and clinical interpretation of 18F-fluciclovine PET/CT images, we would like to share our wealth of knowledge on 18F-fluciclovine PET/CT imaging, with an emphasis on the factors that may affect image quality and interpretation. The purpose of this guide is to provide technologists with best-practice knowledge on 18F-fluciclovine PET/CT imaging for recurrent prostate cancer.
PATIENT SCHEDULING
Currently, the only FDA-approved and reimbursable indication for 18F-fluciclovine PET/CT is restaging in patients with clinical suspicion of recurrent prostate cancer based on a rising PSA level after prior treatment. When ordering the study, both the referring physician and the nuclear medicine physician must ensure that the 18F-fluciclovine PET/CT study is being applied for the appropriate reason to prevent challenges with insurance approval. Aside from the general indication of cancer restaging, any other clinical questions should also be noted. When scheduling the 18F-fluciclovine PET/CT scan, it is essential to remember that amino acid transporters are also upregulated in inflamed cells, although to a lesser extent than in prostate cancer cells (8). At this time, there is no dedicated study evaluating the influence of recent procedures on 18F-fluciclovine uptake, and no official recommendation has been made on the optimal wait time for 18F-fluciclovine imaging after an intervention. However, to keep an optimal tumor-to-background ratio, it is reasonable to schedule the 18F-fluciclovine PET/CT scan at least 2 wk after an intervention to allow time for resolution of any inflammation.
After the appropriate indication is confirmed and a date is scheduled, the 18F-fluciclovine dose needs to be preordered through the central distributer pharmacy website for 18F-fluciclovine. Depending on the regional demand, the order should be placed at least 48 h before the scheduled date. However, this timing can be site-specific.
PATIENT PREPARATION
According to the standardized protocol, patients are recommended to fast for at least 4 h before injection, including water restriction. The altered biodistribution of 18F-fluciclovine in a nonfasting population is not well investigated, compared with 18F-FDG radiotracer. Although it is recommended that nonfasting patients be rescheduled, whether to reschedule the exam should be discussed with the interpreting physician as exceptions may be made. If an exception is made, the interpreting physician should carefully review the image quality. There are currently no known contraindicated medications; therefore, patients can take their prescription medications as usual with sips of water. Patients should engage in no exercise or physical exertion for 24 h before the time of imaging. Excessive exercise may potentially cause increased muscle uptake that could degrade the quality of the images. If possible, patients should be contacted and reminded of these instructions before the day of imaging.
On the day of imaging, the patient’s compliance with the preparation instructions should be evaluated. In clinical experience, patients who voided just before 18F-fluciclovine injection had higher early 18F-fluciclovine bladder excretion compared with those who did not void. Therefore, patients should be advised not to void immediately before injection and imaging (9,10). For centers that perform the PET/CT scan with an oral contrast medium, it is suggested that the patients refrain from voiding for 1 h after administration of the contrast medium until after the 18F-fluciclovine has been injected and the imaging completed (9). For patients with a Foley catheter, no specific intervention is required. The ability to lie still for the duration of imaging (∼30 min) is important to avoid motion artifacts. Therefore, comorbidities that may pose challenges for imaging by preventing the patient from lying flat and any adjustments needed to accommodate for this inability should be communicated to the interpreting physician.
Studies have shown that parameters such as PSA and Gleason score have a positive correlation with the detection of prostate cancer recurrence, with a higher risk of having bone metastasis at PSA levels above 20 ng/mL (11). For 18F-fluciclovine prostate imaging, PSA demonstrated a strong linear correlation with positive findings (12). It is therefore advisable to complete a prostate cancer information questionnaire including details on Gleason score, current and prior lowest PSA values, use of hormonal therapy (current or past), prior therapy (e.g., prostatectomy, radiation therapy, or cryotherapy), known metastatic disease, and availability of prior scans. Having such information can help guide the physician during final image interpretation, especially in equivocal cases.
RADIOPHARMACEUTICAL INJECTION
18F-fluciclovine is a synthetic amino acid radiotracer with a half-life of about 110 min. This allows for same-day delivery of the tracer from a local distributor. Standard radiation safety and radiopharmaceutical administration precautions should be followed during the handling and injection of 18F-fluciclovine. According to the guidelines, the recommended dose per patient is 370 MBq (10 mCi) ± 20%.
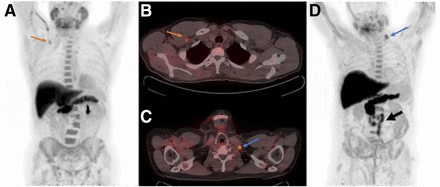
The 18F-fluciclovine is injected intravenously while the patient is lying supine within the PET/CT scanner. Although the mechanism is not well understood, radiotracer uptake may be seen along the injected vein (Figs. 1A and 1B). To minimize this phenomenon, it is recommended that the patient’s arms be down when the 18F-fluciclovine is injected. Injection via the left arm may lead to uptake in the left subclavian vein. The uptake may appear as a local focus of supraclavicular uptake and may mimic the presence of a metastatic left supraclavicular, or Virchow, lymph node. Therefore, it is preferable that 18F-fluciclovine be injected into the right arm. The presence of a supraclavicular metastatic lymph node in prostate cancer is not common but may be seen in rare cases (Figs. 1C and 1D) and should not be confused with vein uptake. After the 18F-fluciclovine injection, it is recommended that an intravenous sterile 0.9% saline flush be administered to ensure full dose delivery. Afterward, the arms are repositioned to above the head. The remaining dose within the syringe is assayed to determine residual activity. The assayed, residual, and net administered dose, as well as the injected site, should be recorded (9). Subsequently, the net administered dose is used for the SUV calculation.
(A) 18F-fluciclovine intravenous injection via right antecubital vein demonstrates increased uptake in right axillary vein on maximum-intensity projection (arrow). (B) Focal uptake in subclavian space may mimic or mask metastatic lymph node uptake on PET/CT transaxial image (arrow). (C and D) In patient injected via right antecubital vein, focus of increased 18F-fluciclovine uptake in left supraclavicular space correlates with enlarged suggestive lymph node on PET/CT transaxial image (C) and maximum-intensity projection (D) (blue arrows). Additional diffuse retroperitoneal metastatic lymph nodes are noted (black arrow).
PATIENT POSITIONING
Unless prevented by the patient’s clinical limitations, the recommended position for imaging is supine with the arms above the head (Fig. 2)
CT scout images demonstrating recommended positioning of patient with arms up (A) and alternative, less preferred, position with arms down (B).
CT ACQUISITION
A CT scan for anatomic correlation and attenuation correction is done per site standard from mid thigh to skull base. The use of intravenous or oral contrast medium for the CT scan is per site standard. However, the density of the contrast medium may result in an attenuation correction artifact (13). If contrast medium is used, the CT images should be obtained after the PET acquisition to minimize the diuretic effect of the medium on 18F-fluciclovine (9). The use of a high-quality CT scan as an attenuation-correction CT scan is not mandatory but is preferable for better characterization of small suggestive structures. To prevent respiration artifacts from rapid breathing, patients should use shallow breathing during both the CT and the PET acquisition (Fig. 3). In challenging cases, a respiration-gated PET/CT acquisition can be done (9).
Respiration artifact from rapid breathing pattern on 18F-fluciclovine PET maximum-intensity projection appears as artificially decreased tracer uptake (arrow) by liver dome.
PET ACQUISITION
Because of the rapidity of 18F-fluciclovine kinetics, the highest ratio of tumor to normal background tissue is seen between 4 and 10 min after injection. Therefore, to maximize the early imaging period, it is recommended that the PET acquisition begin at the mid thigh 3–5 min (target, 4 min) after injection and proceed caudocranially, with the bed positions set such that the prostate gland is in the middle of the first bed position. Setting the bed position as such without excluding part of the lower pelvis can be challenging in some individuals. Hence, having the prostate gland within the middle-to-end of the first frame is also acceptable.
Although the manufacturer guidelines for 18F-fluciclovine advise 5 min per bed position in the pelvis followed by 3–5 min per bed position up to the base of the skull, image acquisition is scanner- and site-dependent. At our center, using a Philips Ingenuity time-of-flight PET/CT scanner, we use 3.5 min per bed position for the first 3 bed positions, followed by 3 min per bed position up to the skull base. However, 2–5 min per bed position may be adequate using time-of-flight or digital PET/CT scanners. For quality purpose, the imaging start and end times must be recorded. The entire imaging procedure is expected to take approximately 25–30 min per patient. In rare situations in which the acquisition is delayed, such as in the case of a claustrophobic patient or a scanner malfunction, the sensitivity of the examination may decrease. Therefore, if the final images do not answer the clinical question, the patient will have to be rescheduled for a repeat study. 18F-fluciclovine PET/CT can safely be repeated 24 h after the prior injection.
A summary of patient preparation, 18F-fluciclovine injection, PET/CT acquisition, and imaging pitfalls is provided in Appendix A.
QUALITY CONTROL
According to the American College of Radiology technical standards, quality control for 18F-fluciclovine PET is no different from that for other types of PET imaging, such as 18F-FDG PET/CT (9).
NORMAL BIODISTRIBUTION OF 18F-FLUCICLOVINE
A detailed description of the normal biodistribution of 18F-fluciclovine has been published (14). The liver and pancreas demonstrate the most intense 18F-fluciclovine uptake, followed by moderate uptake in the marrow, pituitary, and salivary glands. Mild uptake is seen in the muscle, and variable mild to moderate activity is seen in the small bowel. The lowest 18F-fluciclovine activity is in the brain and lung parenchyma (Fig. 4). 18F-fluciclovine is slowly eliminated through the renal system. Therefore, over time, urinary excretion of 18F-fluciclovine into the bladder is expected.
Normal biodistribution of 18F-fluciclovine on PET maximum-intensity projection shows highest uptake within liver and pancreas.
IMAGE INTERPRETATION
A description of 18F-fluciclovine PET/CT image interpretation was previously published (3,9). Additional resources for reader training are available online through the Society of Nuclear Medicine and Molecular Imaging website. Emphasis is placed on the localization of lesions with visually increased uptake (with the assistance of quantitation) to improve the specificity of disease detection. For quantitation evaluation, 18F-fluciclovine uptake is measured as SUVmax within a region of interest drawn on the target lesion. This uptake is compared with the SUVmean of target background structures: distal abdominal aorta (preferably at the same bed position as the lesion), marrow (L3 vertebra), and liver. Any target lesion with visualized uptake greater than that of marrow or liver is considered highly suggestive of malignancy. However, for lesions or lymph nodes less than 1 cm in largest diameter, the SUVmax may be underestimated because of volume averaging. Therefore, subcentimeter lesions are considered suggestive of malignancy if the uptake is significantly higher than that of the blood pool and visually approaching that of marrow.
Because of the relatively higher physiologic uptake of 18F-fluciclovine in the liver and bone marrow, detection of metastatic liver lesions can be challenging (15). Appropriate liver windowing is recommended to improve visualization of metastatic bone and liver disease. For lytic or CT-occult bone lesions, focal intense 18F-fluciclovine uptake is considered suggestive of malignancy. In contrast, dense sclerotic lesions may demonstrate falsely mild to no uptake (1,2,4,16,17). With unpublished experience, we learned that focal intense 18F-fluciclovine uptake within the acetabulum and iliac bones without abnormal CT findings has a high false-positive rate. Hence, in some cases, further evaluation of bone metastasis with MRI and bone SPECT or PET scans should be considered.
18F-FLUCICLOVINE PITFALLS
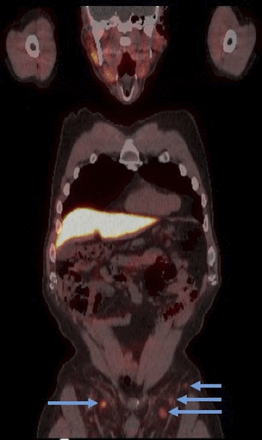
Common findings that may mimic diseases on 18F-fluciclovine PET/CT have also been published (14). Although 18F-fluciclovine has a high detection rate for prostate cancer, nonspecific uptake has been reported in inflammatory and benign processes. Most commonly seen is bilateral uptake in the inguinal lymph nodes (Fig. 5). Since metastasis of prostate disease to the inguinal lymph nodes is highly unlikely, uptake in the inguinal lymph nodes is mostly deemed benign. However, unilateral uptake in an inguinal lymph node may be suggestive in the correct clinical setting. For patients who underwent radiation therapy as their initial management for prostate cancer, diffuse uptake within the treated prostate may also be nonspecific.
18F-fluciclovine PET/CT image demonstrating moderate uptake bilaterally in reactive inguinal lymph nodes (arrows).
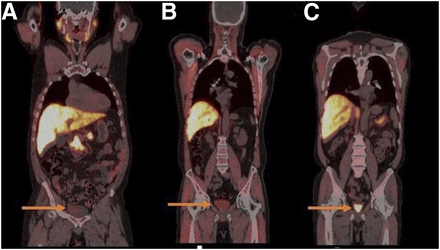
The secretion of 18F-fluciclovine into the ureters may mimic the presence of nodal disease. It is therefore important to identify the course of the ureter to delineate ureteric uptake from true nodal disease. The low washout of 18F-fluciclovine into the bladder over time is ideal for the evaluation of pelvic malignancy such as prostate cancer (8,14). In clinical practice, however, a higher level of bladder uptake has been reported (12). Higher bladder activity is noted in patients who voided just before 18F-fluciclovine injection than in those who did not void (Fig. 6). Hence, it is recommended that patients avoid voiding for at least 30 min to 1 h before 18F-fluciclovine injection (9,12,18,19).
18F-fluciclovine PET/CT coronal images demonstrating mild (SUVmean > blood pool) (A) and moderate (SUVmean > marrow < liver) (B) urine radioactivity in patients who did not void before injection of 18F-fluciclovine, compared with intense urine radioactivity (SUVmean > liver) (C) in patient who voided.
CONCLUSION
18F-fluciclovine is a new FDA-approved PET radiotracer for restaging in patients with suspected recurrent prostate cancer based on a rise in PSA level after local or systemic therapy. As one of the busiest 18F-fluciclovine PET/CT imaging centers, we provide our best-practice guidelines for the performance of 18F-fluciclovine PET/CT to ensure quality images for disease detection. These guidelines include proper patient preparation and positioning, as well as proper 18F-fluciclovine injection and imaging techniques to prevent artifacts and image misinterpretation.
DISCLOSURE
Bital Savir-Baruch has received a grant sponsored by Blue Earth Diagnostics. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We acknowledge Matt Duiven, CNMT, and Don Swatosh, CNMT, for their help with the scout CT images.
APPENDIX A: BEST PRACTICES FOR 18F-FLUCICLOVINE PET/CT
Fluciclovine is an amino-acid–based PET radiotracer that has higher diagnostic performance in prostate cancer than does conventional imaging, with especially higher specificity in the detection of nodal disease in recurrent prostate cancer.
Indications
The agent is FDA-approved for restaging of patients that are suspected to have recurrent prostate cancer based on a rise in PSA level after therapy.
Contraindications
There are no contraindications.
Patient Preparation
Have patient fast for at least 4 h before injection of 18F-fluciclovine. Prescribed medications can be taken with sips of water only.
Have patient avoid exercise or physical exertion 24 h before the time of injection.
Advise patient not to void for at least 1 h before 18F-fluciclovine injection and imaging.
Establish intravenous access, preferably in the right arm.
Radiopharmaceutical Administration
Use a recommended approximately 370 MBq (10 mCi) ± 20%, diluted with 0.9% normal saline up to 10 mL.
Inject intravenously, preferably in the right arm while the patient is supine with arms at sides. After injection, flush with 0.9% normal saline to ensure full dose delivery.
Raise the patient’s arms above the head in a ready position for imaging. If this position is challenging for the patient, the arms can be down.
Image Acquisition
CT.
After injecting the 18F-fluciclovine, acquire the CT scan from mid thigh to skull base for anatomic correlation and attenuation correction.
If intravenous contrast medium is required, the CT scan should be acquired after the PET scan because of the possible diuretic effect of the contrast medium.
PET.
Perform a scout view and set the limits of acquisition from mid thigh to skull base.
Set the bed positions such that the prostate is within the center of the first bed position.
Start imaging 3–5 min (with a goal of 4 min) after 18F-fluciclovine injection to avoid abnormal biodistribution.
Although the recommendation is to acquire images at 5 min per bed position in the pelvis followed by 3–5 min per bed position up to the base of the skull, the acquisition is site- and scanner-dependent.
For quality and accuracy, have a preset 18F-fluciclovine–specific imaging protocol on the PET/CT scanner.
After image acquisition, check images for any errors or artifacts.
Pitfalls
Excretion of 18F-fluciclovine to the urinary bladder poses a diagnostic challenge. Encourage patients to refrain from voiding before the injection of 18F-fluciclovine, to significantly reduce urinary bladder excretion.
For PET/CT with contrast medium, ask patients to refrain from voiding from 1 h before injection until completion of the 18F-fluciclovine PET/CT scan. 18F-fluciclovine uptake by the injected vein wall in the left arm can mimic the presence of a metastatic left supraclavicular node (Virchow node).
Prefer injection into the right arm.
Inject while the patient’s arms are down.
After injection, flush with saline before lifting the arms above the head to an appropriate position for imaging.
Footnotes
Published online Jun. 10, 2019.
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. Complete the test online no later than December 2022. Your online test will be scored immediately. You may make 3 attempts to pass the test and must answer 80% of the questions correctly to receive 1.0 CEH (Continuing Education Hour) credit. SNMMI members will have their CEH credit added to their VOICE transcript automatically; nonmembers will be able to print out a CE certificate upon successfully completing the test. The online test is free to SNMMI members; nonmembers must pay $15.00 by credit card when logging onto the website to take the test.
REFERENCES
- Received for publication February 10, 2019.
- Accepted for publication May 29, 2019.