Abstract
The field of molecular imaging is undergoing a period of expansion. The number of radiopharmaceutical ligands, therapeutic isotope combinations, and targets continues to grow, with several new PET agents being approved over the last few years for diseases such as neuroendocrine tumor and prostate cancer. We will need to work together as a community to effectively use and advance these promising agents.
The field of molecular imaging—the visualization, characterization, and measurement of biologic processes at the molecular and cellular levels in humans and other living systems—is undergoing a period of growth and expansion. Of the 10 Food and Drug Administration (FDA)–approved PET imaging agents, 6 have been approved in the past 5 y (Table 1). This explosion of approvals may be attributed to the release of title 21 of Code of Federal Regulations, part 212 (Current Good Manufacturing Practice for Positron Emission Tomography Drugs), on December 10, 2009. Part 212 provides clear expectations and guidelines for FDA approval of radiopharmaceuticals.
FDA-Approved PET Agents
Of the most recently approved agents, Amyvid (18F-florbetapir; Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly and Co.), Vizamyl (18F-flutemetamol; GE Healthcare), and Neuraceq (18F-florbetaben; Piramal)—all proprietary agents with intellectual property or patents—underwent a typical development pathway. Each molecule was owned by a corporate entity, which guided the development of the agents through prospective phase 1, 2, and 3 studies, including an imaging correlation to postmortem confirmation of β-amyloid neuritic plaques (the plaques that are thought to cause Alzheimer disease). 11C-choline (Mayo Clinic), Axumin (18F-fluciclovine; Blue Earth Diagnostics), and NETspot (68Ga-DOTATATE; Advanced Accelerator Applications [AAA]), however, all took alternate routes to FDA approval.
In September 2012, the FDA approved 11C-choline injection as a radioactive diagnostic agent for PET imaging of patients with suspected prostate cancer recurrence and noninformative bone scintigraphy, CT, or MRI. In these patients, 11C-choline PET imaging may help identify potential sites of prostate cancer recurrence for subsequent histologic confirmation. Suspected prostate recurrence is based on elevated levels of prostate-specific antigen in the blood after initial therapy. A limitation of use specifically noted on the drug label was that 11C-choline PET imaging is not a replacement for histologic verification of recurrent prostate cancer.
At the time of the approval, 11C-choline was a technologic breakthrough in prostate cancer imaging and therapy, detecting sites of recurrence and, subsequently, allowing more effective treatment strategies to be used months earlier than conventional imaging. Unlike the β-amyloid agents, which underwent multiple prospective studies, the safety and effectiveness of 11C-choline was documented with a systematic review of 5 published studies involving 98 patients. The FDA noted that there was a substantial body of human studies with 11C-choline that showed utility in other cancer types as well. Except for minor skin inflammation at the injection site, no adverse events were reported (1).
The approval of choline by the FDA represented a paradigm shift in radiopharmaceutical drug development. This was apparent with the approval of new agents, namely Axumin and NETspot, within 1 wk of each other in May and June 2016. 18F-fluciclovine was developed at Emory University by Mark Goodman, a professor of radiology and imaging scientist at Emory University School of Medicine. The first paper on the compound was published in 1999. Emory patented the compound, licensed it to Japan’s Nihon Medi-Physics, and Goodman continued his research for several years. In 2008, GE Healthcare licensed the technology before spinning it off to the newly formed Blue Earth Diagnostics in 2014 (2). Of note, SNMMI’s Clinical Trials Network (CTN) was collaborating with GE Healthcare on the agent and had secured a grant from Movember to conduct a phase 3 study before the transfer to Blue Earth Diagnostics. Because of this history, both Emory University and CTN played a role in the filing of the new drug application. Axumin was approved on the basis of 2 studies. The data were submitted from 4 clinical sites in the United States, Italy, and Norway and analyzed prospectively by United Kingdom–based Blue Earth Diagnostics. The first study was a comparison of 18F-fluciclovine scans to histopathology data in 105 men; the second was a comparison to 11C-choline scans in 96 men. Both studies supported the indication for imaging with Axumin in men with prostate cancer with elevated prostate-specific antigen levels after prior treatment (3).
The first scans with 68Ga-labeled somatostatin receptors were done in Europe as early as 1999 (4). Literally thousands of articles on the human use of 68Ga-DOTATATE have been published since that time, including many saying that this is a safe and effective method of imaging neuroendocrine tumors (NETs) (5). Because of the success of 68Ga-somatostatin receptor imaging in Europe and Australia, physicians in the United States wanted to study the agents. The CTN started a gallium users group in 2012 to help U.S. institutions conduct 68Ga-DOTATATE and -DOTATOC studies. CTN developed standardized release criteria for the agents, as well as developing protocols, imaging manuals, a template investigational new drug application, and information on gallium generators so that universities had a toolkit to facilitate expanded-access trials. By 2014, the number of sites imaging with 68Ga-somatostatin receptors increased from 2 to 12, offering NET patients in the United States a scan that previously would have required a trip to Europe.
NET is an orphan disease, which the FDA defines as a disease with a prevalence of 200,000 cases or less. Prevalence is the number of cases in the population at a given time, or, simply, how widespread a disease is (all cases of NET irrespective of when each was diagnosed). Incidence, by comparison, is the rate of occurrence of new cases and gives one information about the risk of contracting a disease (how many cases were diagnosed in a particular year). Thus, NETspot was designated an orphan drug and received a priority-review status. Orphan drugs are handled by the FDA’s Office of Orphan Products Development and have a slightly different pathway to approval, including the waiving of the nearly $2 million fee authorized by the Prescription Drug User Fee Act to file the new drug application. Three studies established the safety and effectiveness of NETspot: a comparison of 68Ga-DOTATATE to CT or MRI, a histopathology or clinical follow-up comparison, and an evaluation of patients with NET recurrence (6).
In the approval of these 3 agents, FDA accepted an analysis of imaging data that were collected outside the confines of a company-sponsored phase 3 trial. This points to the importance of these agents in helping determine effective treatment strategies in serious diseases.
USING THE NEW RADIOPHARMACEUTICALS
In 2012, the Mayo Clinic was the only site approved to use 11C-choline. Since that time, several other institutions—the University of Texas M.D. Anderson Cancer Center, the Washington University School of Medicine in St. Louis, and Zevacor—have received FDA approval after submission of abbreviated new drug applications. Zevacor produced choline at its Decatur, Illinois, facility with plans to expand to additional sites. (Zevacor was acquired by Sofie Biosciences in May 2017.) With a half-life of 20 min for 11C, distribution is extremely limited. Administration differs from 18F-FDG in that patients do not need to fast. The 370- to 740-MBq (10–20 mCi) dose is injected as an intravenous bolus while the patient is on the scanner bed, and imaging begins immediately afterward. Localized uptake of 11C-choline in a site suggestive of prostate cancer recurrence is determined by comparison of the anatomic relationship of concentrated radioactivity to the neighboring tissue background (exclusive of radioactivity that accumulates physiologically in the pancreas, liver, spleen, kidney, and colon) (Fig. 1).
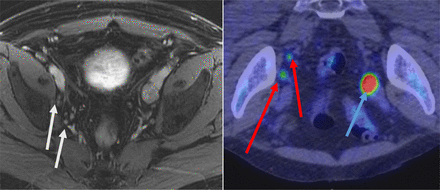
MRI (left) and 11C-choline PET/CT (right) images of patient with prostate cancer recurrence (prostate-specific antigen level, 3.3 ng/mL) show enlarged left external iliac lymph node with 11C-choline uptake (blue arrow) and small right external iliac and internal iliac lymph nodes (white arrows) with 11C-choline uptake (red arrows). The small right nodes are considered abnormal only on 11C-choline PET/CT. Disease was more extensive on PET than on MRI. After treatment, all nodal disease showed resolution and prostate-specific antigen level was less than 0.01 ng/mL. (Courtesy of Mayo Clinic.)
Axumin, labeled with 18F (half-life, 110 min), is manufactured and distributed in the United States by an ever-expanding number of PETNET Solutions radiopharmacies. Fluciclovine is an amino acid; it can image prostate cancer and other types of cancer because amino acids are key nutrients for tumor growth and the 18F-fluciclovine is therefore incorporated into the tumor cells (7). In preparation for a 18F-fluciclovine PET scan, patients should avoid significant exercise for at least 1 d beforehand (to avoid amino acid uptake in muscle) and should not eat or drink for at least 4 h beforehand. Patients are dosed on the scanner bed with 370 MBq (10 mCi) by intravenous bolus injection. The uptake time is 3–5 min, with a target of 4 min. Imaging should start over the pelvis with a least 3 min per bed position; the scan time for the first 2 bed positions can be increased if desired. Localization of prostate cancer recurrence in sites typical of prostate cancer is based on 18F-fluciclovine uptake in comparison to background tissue uptake. For lesions less than 1 cm in diameter, focal uptake greater than blood pool uptake should be considered suggestive of prostate cancer. For larger lesions, uptake equal to or greater than bone marrow uptake is considered suggestive of prostate cancer recurrence. To be qualified to order and read Axumin scans, physicians must successfully complete the CTN-developed Axumin Image Interpretation Training presentation and examination (http://www.snmmi.org/Research/Content.aspx?ItemNumber = 10689&navItemNumber = 6820) (Fig. 2).
CT, 18F-fluciclovine PET, and PET/CT (left to right) images of prostate cancer patient presenting with rising prostate-specific antigen level (2.31 ng/mL) after external-beam radiation therapy and brachytherapy. MRI was negative for extraprostatic disease. Images show positive subcentimeter-sized right common iliac and obturator nodes (arrows), which were found to be malignant on laparoscopic dissection.
AAA’s NETspot, 68Ga-DOTATATE, has a half-life of 68 min and is distributed in the United States in unit dose form by Cardinal Health and United Pharmacy Partners. Sites that have their own 68Ge/68Ga generator, namely the Eckert and Ziegler GalliaPharm generator, can order lyophilized kits from AAA and prepare NETspot onsite. (Of note, only the good manufacturing practice–grade Eckert and Ziegler generator GalliaPharm is approved for use with the kits.) Patients are advised to drink enough water to ensure adequate hydration before receiving 68Ga-DOTATATE; no other special preparation is required. There is controversy over the temporary discontinuation of cold octreotide therapy before the scan. For patients on long-acting therapy (Sandostatin LAR Depot [octreotide acetate; Novartis] or Somatuline Depot [lanreotide; Ipsen), the scan should be scheduled at the end of the treatment cycle (e.g., 4 wk after the last injection). For short-acting Sandostatin, the scan should take place 12 h or more after the last administration. The recommended dose of radioactivity is 2 MBq/kg of body weight (0.054 mCi/kg) up to 200 MBq or 5.4 mCi. The uptake time is 40–90 min, with a goal of 60 min. The typical field of view is mid thigh to top of head, with the direction of imaging being caudal to cranial at a suggested rate of 3–4 min/bed position, depending on the model of the scanner and the body habitus of the patient. If a diagnostic CT scan is requested as part of PET/CT, the CT protocol appropriate for the body regions requested should be used. Intraluminal gastrointestinal contrast medium may be used, but a positive oral contrast material such as barium should be avoided as it may cause attenuation correction defects (8). 68Ga-DOTATATE binds to all somatostatin receptor type 2–expressing cells, including the pituitary, thyroid, liver, adrenal glands, spleen, pancreas, bowel, and urinary system. A complete NETspot image interpretation training course, developed by CTN, is available on the SNMMI website (http://www.snmmi.org/Research/Content.aspx?ItemNumber = 10689&navItemNumber = 6820) (Fig. 3).
Three levels of 68Ga-DOTATATE PET/CT (left) and maximum projection PET on right. 73-y-old woman with history of metastatic NET. Lung and lymph node metastases (yellow arrows), multiple liver metastases, and terminal-ileum primary tumor (blue arrows) are seen. (Courtesy of University of California, San Francisco.)
Currently, the Centers for Medicare and Medicaid Services reimburse the following 3 agents. 11C-choline is reimbursed at $5,700 per study dose up to 740 MBq (20 mCi). 18F-fluciclovine is reimbursed at $389.55 per 37 MBq (1 mCi) administered. 68Ga-DOTATATE is reimbursed at $66.74 per 3.7 MBq (0.1 mCi) administered (9). Coverage by private payers varies; SNMMI continues to work for consistent reimbursement for these and all radiopharmaceuticals.
WHAT’S NEXT?
Lantheus, in partnership with GE Healthcare, is starting a 522-patient international trial to evaluate the diagnostic efficacy of 18F-flurpiridaz (Lantheus) myocardial perfusion PET in the detection of coronary artery disease. This would be the second phase 3 trial for 18F-flurpiridaz on its path to regulatory approval and commercialization.
Perhaps the hottest topic in molecular imaging is theranostics, a combination of a diagnostic agent and a therapeutic agent, such as 68Ga- and 177Lu-DOTATATE. The phase 3 randomized, controlled NETTER-1 trial showed the remarkable efficacy of 177Lu-DOTATATE (Lutathera; AAA) for the treatment of patients with advanced midgut NETs. The study demonstrated markedly longer progression-free survival and a significantly higher response rate than with long-acting release high-dose octreotide (10). The FDA provided a date of January 26, 2018, for approval of the new drug application (11). The other theranostic combination dominating the current literature is 68Ga- and 177Lu-labeled prostate-specific membrane antigen for prostate cancer imaging and therapy. In Europe, prostate-specific membrane antigen has been labeled with α-emitting 225Ac and used to treat castration-resistant prostate cancer, with promising antitumor activity (12). Another promising prostate cancer theranostic pair that bears mentioning are the bombesin analogs, 68Ga- and 177Lu-RM2. Pentixafor, labeled with 99mTc or 68Ga, binds avidly to CXC chemokine type 4 receptors, which are upregulated in several cancers, most notably multiple myeloma. The therapeutic analog pentixather, labeled with either α- or β-emitting particles, has shown promising activity against both hematologic malignancies and solid tumors (13). Although none of these imaging or therapy agents are FDA-approved, all are being studied in the United States and abroad.
These promising personalized, precision medicine combinations are garnering attention outside the nuclear medicine world. The Swiss drug maker Novartis is buying AAA for $3.9 billion to strengthen its oncology portfolio with Lutathera and other theranostic agents in the pipeline (14). Endocyte, an Indiana-based biopharmaceutical company, announced its acquisition of exclusive worldwide licensing rights to prostate-specific membrane antigen 617, a therapeutic ligand for prostate cancer when labeled with 177Lu. Endocyte estimates that radiotherapy for prostate cancer offers a $1 billion market opportunity (15).
CONCLUSION
The radiopharmaceutical drugs discussed in this article represent the tip of the iceberg, as the number of ligands, therapeutic isotope combinations, and targets continues to grow. As a community, we will need to work together to effectively use and advance these promising agents.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Feb. 2, 2018.
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. Complete the test online no later than March 2021. Your online test will be scored immediately. You may make 3 attempts to pass the test and must answer 80% of the questions correctly to receive 1.0 CEH (Continuing Education Hour) credit. SNMMI members will have their CEH credit added to their VOICE transcript automatically; nonmembers will be able to print out a CE certificate upon successfully completing the test. The online test is free to SNMMI members; nonmembers must pay $15.00 by credit card when logging onto the website to take the test.
REFERENCES
- Received for publication November 9, 2017.
- Accepted for publication December 29, 2017.