Abstract
18F-FDG PET/CT is a valuable noninvasive tool in oncologic imaging, and its application in the diagnosis of liver metastases has been very convincing. Both the sensitivity and the specificity of 18F-FDG PET/CT are high for detecting liver metastases from various tumors including colorectal, breast, and lung. Such liver metastases are typically 18F-FDG–avid. We present atypical 18F-FDG PET findings in a lung cancer patient with known liver metastases and PVT.
Oncology patients are prone to thrombotic complications because of their hypercoagulable state. Our case report describes a patient with lung adenocarcinoma, liver metastases, and portal vein thrombosis (PVT). We present interesting PET and CT findings highlighting the lack of significant 18F-FDG uptake in liver metastases as well as demonstrating a geographic uptake pattern congruent with decreased hepatic attenuation from sequelae of PVT.
CASE REPORT
A 50-y-old man had stage IV lung adenocarcinoma, osseous metastases on bevacizumab (Avastin; Genentech) chemotherapy, and systemic anticoagulation for left calf vein thrombosis. He had a progressive increase in levels on liver function tests (alkaline phosphatase 260 [normal, 39–117], alanine transaminase 356 [normal, 15–58], aspartate transaminase 197 [normal, 14–40], lactate dehydrogenase 431 [normal, 120–240], and normal total bilirubin).
CT abdomen/pelvis (portal phase) demonstrated geographic areas of decreased attenuation involving the entire left hepatic lobe and segments of the posterior right hepatic lobe that were not consistent with metastases. No liver lesions suggestive of metastases were seen. The left hepatic lobe perfusion abnormality appeared secondary to left PVT. The posterior segmental abnormality was also attributed to a small-vessel portal occlusion, although no definite thrombus was seen.
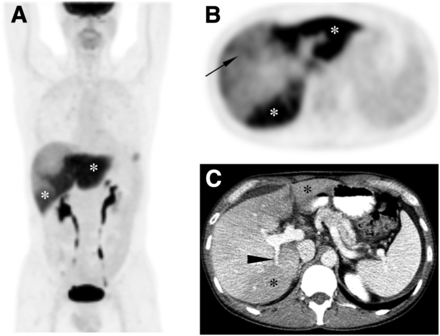
PET/CT images (Fig. 1) revealed congruent intense hypermetabolic activity within the areas of hypoattenuation within the liver, suspected to be from PVT-related parenchymal inflammation or injury. Within the anterior hepatic segment, there were patchy areas of mild 18F-FDG uptake atypical of metastases. Rising liver function levels led to ultrasound-guided biopsy of this area, which revealed metastatic pulmonary adenocarcinoma. For progressive liver metastatic disease and PVT, his chemotherapy was modified, with good response and improved liver function. Follow-up CT demonstrated left hepatic lobe atrophy and cavernous transformation, sequelae of PVT.
(A and B) Maximum-intensity-projection image of 18F-FDG PET/CT (A) and axial PET image (B) demonstrate geographic intense hypermetabolic activity within left hepatic lobe and posterior segment of right hepatic lobe (white asterisks) from PVT. Patchy uptake of 18F-FDG is noted in anterior hepatic segment (black arrow), which is atypical for metastases. (C) Axial contrast-enhanced CT (portal phase) shows congruent areas of hypoattenuation (black asterisks) and abrupt termination of left portal vein (white arrowhead) and posterior right portal vein (black arrowhead) from thrombosis. Incidental focal 18F-FDG activity in left lower chest on maximum-intensity-projection image was from pulmonary inflammation.
DISCUSSION
18F-FDG PET has been shown to be highly sensitive in detecting hepatic metastases from different primary tumors, seen most often as hypermetabolic lesions. PET/CT was shown to be superior to contrast-enhanced CT in the detection of untreated hepatic metastases in a prospective study evaluating 45 patients with various primary cancers. The sensitivity and specificity for the detection of hepatic metastases were 87.9% and 16.7%, respectively, for contrast-enhanced CT, and 97% and 75%, respectively, for PET/CT (1). However, not all hypermetabolic lesions are malignant. For example, abscesses, infections, and benign neoplasms such as adenomas and hemangioendotheliomas may be 18F-FDG–avid (2). A few 18F-FDG PET–negative hepatic metastases similar to those of our patient have also been reported in the literature and were due to either the small size of the lesion or the lower resolution of PET when used alone (3).
Our patient also had PVT, which could represent either a bland blood thrombus that is metabolically inactive or a tumor thrombus that is metabolically active. Common predisposing conditions for PVT include primary or secondary hepatobiliary malignancies, cirrhosis, abdominal sepsis, myeloproliferative disorders, and inherited thrombophilias. The mechanisms involved in the development of a neoplastic venous thrombosis include direct vascular invasion, compression by tumor mass, or the underlying hypercoagulable state.
Characteristic contrast-enhanced CT findings associated with PVT include lack of luminal enhancement, increased hepatic enhancement in the arterial phase, and decreased enhancement in the portal venous phase (4). Published literature supports the role of 18F-FDG PET/CT in differentiating 18F-FDG–avid tumor thrombus from a benign or bland thrombus that is not 18F-FDG–avid (5).
CONCLUSION
Interpretation of 18F-FDG uptake within the liver in patients with more than one pathologic condition can be challenging. In our patient, PET/CT was false-negative for liver metastases but demonstrated an interesting pattern of hypermetabolic activity in the hypoattenuating areas of the liver from PVT.
Footnotes
Published online Feb. 5, 2015.
REFERENCES
- Received for publication December 5, 2014.
- Accepted for publication January 23, 2015.