Abstract
Cardiac CT (CCT) is rapidly evolving as a noninvasive imaging modality. Newer technologic developments in CCT allow the comprehensive assessment of cardiovascular anatomy, including the coronary arteries. There are special considerations regarding obtaining and accurately interpreting CCT studies. On completion of this article, the reader should be able to describe the issues related to adequate patient preparation and acquisition and interpretation of CCT studies, recognize specific limitations that impair image quality and subsequently the accuracy of diagnosis, and list the current indications and future potential applications of this technique.
Coronary artery disease (CAD) remains the most common cause of morbidity and mortality in developed countries. Noninvasive imaging for the detection of CAD has evolved significantly over the last 50 y. Technologic advances have led to the development of newer CT systems with a substantial increase in spatial and temporal resolution as well as a shortening of the imaging acquisition time, making it possible to visualize the beating heart. This is why cardiac CT (CCT) has gained popularity for the detection and quantification of CAD (1–3). By far, CCT is the fastest-growing noninvasive diagnostic cardiac imaging modality in the United States. The percentages of uninterpretable CCT studies have gradually decreased from 20%–40% with 4-slice systems to 15%–25% with 16-slice systems and are now as low as 3%–10% with 64-slice systems. Here we review the technical considerations that are germane to operating a CCT program, including patient preparation, factors that affect interpretation, and current indications and potential applications of this rapidly evolving technique.
PRESCAN CONSIDERATIONS
The most common indication for CCT is the anatomic evaluation of the coronary arteries for the presence of CAD. Before each procedure, it is important to review a patient's medical history to collect relevant clinical data that are crucial for the correct performance and interpretation of the test. Patient information required before CCT includes the following:
clinical history (symptoms such as chest pain and dyspnea);
history of allergies (e.g., iodinated contrast material and medications);
history of asthma or hyperthyroidism;
history of renal disease or multiple myeloma (recent creatinine level);
previous diagnostic examinations (stress test, electrocardiogram [ECG], and echocardiogram).
Heart Rate Control
Patient selection for CCT coronary angiography is critical. A stable, low heart rate is required at the time of the procedure, because motion artifacts can occur, given the current limitations in the temporal resolution of existing scanners. Most studies have demonstrated that the highest image quality for current CCT scans is achieved at heart rates of less than 65 beats per minute (bpm) (4). In most patients with heart rates below 70 bpm, the best phase free of motion is centered on 75% of the R-R interval, corresponding to the diastasis phase of diastole (5). At higher rates, diastasis disappears; therefore, image reconstruction at about 40%–50% of the cardiac cycle is preferred. Nevertheless, there is significant patient-to-patient variability, and often several phases reconstructed at intervals of 5%–10% need to be examined.
Oral or intravenous β-blockers should be administered before the study, aiming for a resting regular heart rate of 50–55 bpm. β-Blockers help reduce heart rate variability during the scan, and for that reason, we recommend their administration almost routinely unless they are contraindicated (e.g., patients with asthma). In such situations, diltiazem or verapamil may be used as an alternative agent, although these drugs are not as effective as β-blockers.
Image quality is often suboptimal in patients with irregular heart rates. Scanning patients during atrial fibrillation should be avoided unless there are relatively stable R-R intervals. Similarly, strong consideration should be given to aborting a scan if frequent ventricular ectopy is present.
Breath Holding
During the test, a breath hold of 15–20 s will need to be performed. If the patient cannot hold still and follow breathing instructions, he or she should not be scanned. Breathing during the scan significantly compromises image quality and produces segments that cannot be evaluated. Before the scan, practicing breath holding helps to avoid such artifacts (Fig. 1).
(A) CCT image obtained from patient who was breathing during image acquisition. Note “stair-step” artifacts, with displacement of trajectory of coronary vessels and chest wall (arrows). (B) 3-Dimensional volume-rendered CT image reconstruction of whole heart. Motion artifacts (arrows) are seen in patients who experience multiple extrasystolic beats during image acquisition.
Iodinated Contrast Material Injection
Because intravenous iodinated contrast material is needed to visualize the coronary arteries, CCT is contraindicated in some subjects with severe contrast material allergy. Subjects with a history of mild contrast material allergy should be premedicated with steroids and antihistamines before the test. Diabetic patients taking metformin should discontinue the use of this drug for days after the test to reduce the risk of lactic acidosis. The relative risk of contrast nephropathy needs to be considered before a contrast-enhanced CCT study, and such a study should be avoided in patients with serum creatinine levels of greater than 1.8 mL/dL. In such situations, the risk and utility of CCT need to be compared with those of alternative diagnostic tests. An invasive diagnostic coronary angiogram may be performed in many cases with a smaller amount of contrast material (30–50 mL) and thus may be a preferred option for patients with renal insufficiency. The contrast medium guidelines provided by the American College of Radiology are a good tool for achieving knowledge of the correct use of iodinated contrast medium and the management of complications (6).
Radiation Dosing
CCT, like invasive angiography, involves radiation exposure. The effective dose, expressed in millisieverts, depends on multiple factors, including the volume of acquisition, the duration of the scan, and the radiation energy level used. The volume of acquisition is typically 12–16 cm for coronary angiography and 18–25 cm for angiography of coronary bypass conduits. The radiation energy level required to obtain adequate image quality depends on the width of the patient's chest, the type of study, and the desired spatial resolution. Current CCT systems with 64 detectors provide a typical dose range on the order of 8–20 mSv for coronary angiography. Corresponding doses are 2–6 mSv for invasive angiography, 10–27 mSv for rest–stress nuclear myocardial perfusion imaging studies, and 3.6 mSv from yearly background radiation exposure. It is estimated that the risk of cancer may increase by 1 in 2,000 CCT studies, depending on the age of the patient. Because of these concerns, the routine application of CCT as a screening test is not justified until more outcome data become available. One strategy that minimizes the effective dose in CCT is dose modulation which, depending on heart rate, may reduce total radiation exposure up to 50%. Another recent strategy developed by some manufacturers involves step-and-shoot acquisition in a nonhelical mode; preliminary data suggest that in selected patients, the dose may by reduced to a total of 2–4 mSv.
CCT in very obese patients (body mass index of >40 kg/m2) should not be considered as a better alternative to invasive angiography because of the significant increase in the effective dose of radiation needed to maintain image quality.
SCAN ACQUISITION
Once an adequate heart rate is achieved, if there are no contraindications (such as severe hypotension or use of phosphodiesterase inhibitors), sublingual nitrates are given to vasodilate the coronary vessels. The acquisition starts with a scout scan (planar x-ray mode), which is used to select the region of interest (usually from the carina to slightly below the diaphragm, but from the subclavian artery down if an internal thoracic graft is be assessed). In many centers, a calcium score scan (no contrast material) is then acquired during a breath hold. A remotely controlled dual-injection system capable of administering iodinated contrast material and saline separately is used for the contrast-enhanced study. Image acquisition may be triggered manually or automatically when the concentration of contrast material reaches a prespecified Hounsfield unit (HU) attenuation value (100–150 HUs) in the descending aorta. Alternatively, before the scan, a timing bolus of 20 mL of contrast medium may be injected at the same rate as that to be used for the scan, followed by a single-slice axial image acquisition every 2 s at the level of the carina. A time–density curve is created by plotting the attenuation values obtained in the descending aorta, and the interval from the onset of injection to the peak of this curve is selected to determine the scan delay. Next, the entire heart volume is scanned within a single breath hold (15–20 s).
IMAGE RECONSTRUCTION AND ANALYSIS
CCT provides complex and detailed 3-dimensional datasets, which are reconstructed from the raw data file according to specific phases of the cardiac cycle.
Once the best phase for analysis is determined, examination of each vessel is performed by use of axial images and multiplanar reconstructed images in any orientation (coronal, sagittal, or oblique). Evaluation of the images in the axial projection is done first, because it represents the data in the form that is acquired and is less prone to reconstruction artifacts. Careful adjustment of image window parameters is done to differentiate the iodine-enhanced lumen from calcified and noncalcified plaques. Other postprocessing formats are also used for assessing cardiac structures. Maximum-intensity-projection images allow the evaluation of longer segments of coronary vessels, but they are limited by overlap from adjacent structures. Three-dimensional volume-rendered images are useful for assessing the relationships among different anatomic structures. Curved multiplanar images are reformatted on a plane to fit a curve (usually the path of a coronary artery) and allow display of the entire vessel in a single image (Fig. 2).
(A) Axial image showing left main coronary ostium and its divisions into left anterior descending, ramus intermedius, and left circumflex arteries. (B) Multiplanar reconstructed image. (C) Anatomic 3-dimensional volume-rendered image showing relationships among left main artery, branches, and adjacent cardiac structures. (D) Curved multiplanar reconstruction of entire length of left circumflex artery. A = aorta; CX = left circumflex coronary artery; LA = left atrium; LAD = left anterior descending coronary artery; LM = left main artery.
CLINICAL APPLICATIONS
Calcium Scoring
The most widely used measure of calcium burden is the calcium score (often known as the Agatston score), which is based on the radiographic density–weighted volume of plaques with attenuation values of greater than 130 HUs. The presence of coronary calcification is a robust predictor (for a calcium score of >100, the risk ratio = 1.88) of adverse cardiovascular events, and the prognostic value of coronary calcium burden has been clearly established (7). Although the utility of screening asymptomatic individuals remains controversial, several studies have indicated that the calcium score provides prognostic information independent of conventional risk factors. In a recently published study, a calcium score of greater than 300 was associated with a significant increase in cardiac events compared with that determined by a clinical score alone (8), supporting the notion that a high calcium score can modify predicted risk; this is especially true for patients in the intermediate-risk category, for whom clinical decision making is most difficult. Patients determined to be at low risk by clinical criteria, however, appear to derive minimal additional prognostic benefit from calcium scoring. These conclusions are represented in a clinical consensus document recently issued by the American College of Cardiology and the American Heart Association (9).
Coronary Angiography
One of the most unique applications of CCT is coronary angiography. Several single-center studies investigated the accuracy of 16-channel CCT coronary angiography for the detection of coronary artery stenosis in patients with known or suggested coronary artery disease and referred for invasive coronary angiography (10–20). In these single-center studies, the sensitivity of CCT coronary angiography ranged from 72% to 95% per coronary segment and from 85% to 100% when each patient was used as the denominator unit. The specificity was reported to be between 86% and 98% per segment and between 78% and 86% per patient. Positive predictive values ranged from 72% to 90% per segment and from 81% to 97% per patient, and negative predictive values ranged from 97% to 99% and from 82% to 100%, respectively.
An important advantage of the newer 32-, 40-, and 64-channel CCT systems is their greater craniocaudal coverage per rotation, which allows shorter breath holds and, consequently, smaller contrast injection volumes, fewer artifacts related to patient breath-hold compliance, and less heart rate variability (21–24). Studies have shown that the superior performance characteristics of 64-slice CCT in terms of spatial and temporal resolution lead to measurable improvements in image quality (25). Recent studies reported sensitivities and specificities of 73%–95% and 95%–99%, respectively, for 64-slice scanners (26–28). The number of segments that cannot be evaluated has been reduced to below 10% in most studies, a finding also representing a significant improvement over the performance of older scanners (Fig. 3).
Oblique coronal image obtained from patient with anginal symptoms and indeterminate stress test results, showing severe stenosis of ostium of left main coronary artery (arrow).
Evaluating coronary artery stenosis in patients with extensive coronary artery calcifications may be difficult and represents a major limiting factor. Reconstructions involving calcified structures tend to overestimate the volume set representing calcium (“blooming”) because of partial-volume averaging effects, which suggest that much of the coronary lumen is apparently occupied by calcified plaque. In addition, the true lumen results in a low-density area because of beam-hardening artifacts. In some situations, it can be difficult to distinguish these artifacts from noncalcified coronary plaque. Because symptomatic patients with very high calcium scores have a very high probability of having obstructive CAD, it is reasonable to avoid CCT coronary angiography and proceed directly to invasive catheterization in these patients (Fig. 4A).
(A) Axial image obtained at level of origin of left main artery, showing extensive calcification in left anterior descending coronary artery. Aortic mechanical prosthetic valve is visualized (arrow). (B) Maximum-intensity-projection image obtained from patient with “kissing” stents in left anterior descending coronary artery and first diagonal branch. In this case, it is difficult to evaluate lumen because of metallic artifacts. Vessels distal to stents are widely patent. A = aorta; CX = left circumflex coronary artery; D1 = first diagonal branch; LAD = left anterior descending coronary artery.
A special consideration is the use of this technique in emergency departments. Several recent studies examined the role of CCT in the evaluation of acute chest pain in patients at low risk for acute coronary syndrome (29–32). In most of these patients, CCT can reliably exclude obstructive CAD and can help to diagnose patients with other potentially life-threatening etiologies of chest pain (such as acute aortic dissection or pulmonary embolism). However, further studies are needed to determine the safety and cost-effectiveness of CCT compared with those of other imaging modalities in this setting.
Atherosclerotic Plaque Volume and Morphology
Recent studies evaluated the feasibility of CCT for quantifying atherosclerotic coronary plaques and differentiating calcified from noncalcified lesions on the basis of their x-ray attenuation. The sensitivity of CCT is greater for calcified (94%) than for mixed (78%) or soft (53%) plaques and is mostly limited to large-caliber vessels. Compared with intravascular ultrasound, CCT tends to underestimate the noncalcified plaque volume but to overestimate the calcified plaque volume. A recent study reported a moderate correlation for the mean plaque area defined by intravascular ultrasound and 64-slice CCT (r = 0.73) (22).
Evaluation of Coronary Stents
Accurate assessment of coronary vessels that have stents remains an important limitation of CCT coronary angiography (33,34). The ability to evaluate the lumen of vessels with stents depends on the type and the diameter of the stent. Practical delineation of in-stent restenosis remains difficult for stents smaller than 3 mm in diameter; the luminal diameter is often underestimated because of partial-volume averaging and blooming artifacts (Fig. 4B). Dedicated postprocessing sharp reconstruction filters, “kernels,” may be helpful in improving the resolution of the stent lumen in some situations (35).
Evaluation of Coronary Artery Bypass Grafts
Other potential indications of CCT include the evaluation of bypass grafts. Grafted vessels have larger calibers and are less prone to motion artifacts than native coronary arteries. The technique is particularly precise in differentiating patent versus totally occluded vessels, with reported sensitivity, specificity, and positive and negative predictive values of 96%, 95%, 81%, and 99%, respectively (36). However, accuracy for the evaluation of distal anastomoses is lower. Metallic artifacts caused by surgical clips may limit the assessment of segments of internal thoracic grafts. Analysis of native vessels is often more difficult in patients who have received coronary artery bypass grafts because of poor distal vessel opacification, more extensive calcification, and smaller lumen size (Fig. 5).
Three-dimensional volume-rendered oblique sagittal view obtained from patient with previous bypass surgery. Arrow indicates distal anastomosis of aortocoronary bypass graft to left anterior descending artery.
Coronary Anomalies
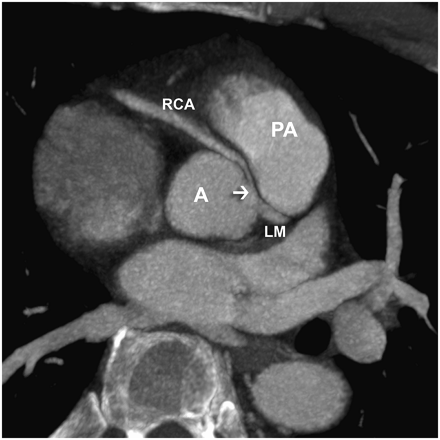
CCT coronary angiography is very useful in evaluating the origin and course of anomalous coronary arteries (37,38). In addition, CCT can easily determine the 3-dimensional relationship of anomalous coronary arteries with the aorta and pulmonary arterial trunk. Also, it can detect aneurysms on coronary vessels, arteriovenous fistulae, and myocardial bridges (Fig. 6).
CCT image obtained for young patient with chest pain. Arrow indicates anomalous origin and course of right coronary artery between aorta and pulmonary arterial trunk. A = aorta; LM = left main artery; PA = pulmonary artery; RCA = right coronary artery.
CCT in Electrophysiology
At present, with the use of pulmonary vein isolation procedures for atrial fibrillation, CCT allows evaluation of the left atrial and pulmonary vein anatomy; establishment of the anatomic position of the esophagus to avoid its perforation during the procedure; and detection of pulmonary vein stenosis after the procedure, which varies according to the experience of the operator and the technique but may be as high as 20%, as detected by CCT (Fig. 7A) (39).
(A) Axial view showing normal anatomy of 4 pulmonary veins and left atrial appendage clear of thrombus (asterisk). (B) Axial view from another patient undergoing evaluation before radiofrequency ablation of atrial fibrillation. Thrombus (asterisk) is visible in left atrial appendage. A = aorta; LA = left atrium; PA = pulmonary artery; PV = pulmonary vein.
Cardiac Masses and Pericardial Disease
CCT is a useful imaging modality for the evaluation of cardiac masses, particularly to determine their location, extent, and anatomic relationships (40). CCT provides superior resolution for detecting calcification, evaluating perfusion, and determining relationships to noncardiac structures.
CCT has high sensitivity for the detection of left atrial appendage thrombi but reduced specificity because it is often difficult to differentiate reduced opacification attributable to slow flow from the actual presence of a thrombus (Fig. 7B).
CCT may accurately determine pericardial thickness and tissue characteristics. Although thickening (>2 mm) and calcification of the pericardium can suggest constriction, CCT is limited to establishing the presence of constrictive physiology. Echocardiography and MRI are superior in this regard.
CONCLUSION
Although CCT is experiencing exponential growth, with an increasing number of applications, the health community is working on defining appropriateness criteria for the correct use of this technique (41), as we try to summarize here. Appropriate indications for CCT are as follows:
chest pain: intermediate pretest probability for CAD (ECG cannot be interpreted or patient is unable to exercise), persistent chest pain after equivocal stress test, or suggestion of coronary anomalies;
acute chest pain in emergency department: intermediate pretest probability for CAD (no changes in ECG and negative enzyme test results);
pulmonary vein isolation, biventricular pacemaker implantation, or coronary arterial mapping in repeat cardiac surgery;
cardiac masses or pericardial disease with technically limited images from echocardiogram, MRI, or transesophageal echocardiography;
complex congenital heart disease: assess anatomy.
Uncertain indications for CCT are as follows:
chest pain: intermediate pretest probability for CAD (ECG can be interpreted and patient is able to exercise) or low or high pretest probability for CAD (no changes in ECG and negative enzyme test results);
acute chest pain: rule out obstructive CAD, aortic dissection, and pulmonary embolism if the pretest probability for one of them is intermediate;
high risk of CAD in asymptomatic patients;
chest pain after revascularization (percutaneous intervention or coronary artery bypass grafts): evaluate bypass grafts or history of revascularization with stents;
intermediate perioperative risk of cardiac events in patients undergoing intermediate- or high-risk noncardiac surgery;
valvular disease (native or prosthetic valves) with technically limited images from ECG, MRI, or transesophageal echocardiogram.
The indiscriminate use of CCT in low-risk populations is not justified, given the risks associated with ionizing radiation and intravenous iodinated contrast material. As previously discussed, patient selection is very important for achieving adequate results. Image quality can compromise diagnostic accuracy in patients with irregular or fast heart rates, morbid obesity, severe calcifications, or coronary stents. New scanners with acquisitions of up to 256 slices per rotation or multiple x-ray tubes have been developed (42,43) to seek to improve temporal and spatial resolution and reduce artifacts.
Acknowledgments
The authors acknowledge Araceli Perez, James Stephens, and Souffrant Milford, cardiac CT technologists at the Radiology Department of Mount Sinai Medical Center. Susanna Prat-Gonzalez is a research fellow funded by a Fundacion CajaMadrid Fellowship.
Footnotes
-
↵* NOTE: FOR CE CREDIT, YOU CAN ACCESS THIS ACTIVITY THROUGH THE SNM WEB SITE (http://www.snm.org/ce_online) THROUGH MARCH 2010. Those without Internet access can obtain a hard copy of the test by calling 703-708-9000, ext. 1247.
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- Received for publication April 3, 2007.
- Accepted for publication September 2, 2007.