Abstract
Objective: The activity of injected radiopharmaceuticals in nuclear medicine, including β-emitters used for pain palliation, has to be monitored systematically. The objective of the present work was to evaluate the situation and precision of activity monitoring for β-emitters in Swiss nuclear medicine laboratories.
Methods: A questionnaire about the monitoring methods used was sent to 50 centers. On the basis of the questionnaire results, an intercomparison of activity measurements with 90Y and 169Er sources was organized.
Results: This study showed that most laboratories check β-emitter activity with a dose calibrator measurement in the original vial provided by the producer or in the injection syringe. They therefore need to have calibration factors for the corresponding measurement geometries. The results of the intercomparison were disappointing overall. Sixteen of 27 90Y measurements and 17 of 22 169Er measurements in the original vial deviated from the reference activity by more than 20%. The situation was similar for the syringe. These discrepancies did not stem from the intrinsic limitation of the measuring method but were mainly attributable to the poor quality of the calibration factors provided by the manufacturers, in addition to lack of follow-up and incorrect background subtraction, particularly for 169Er, by the nuclear medicine laboratories. Manufacturers are being contacted to discuss possible improvements for the situation.
Conclusion: This study showed that commercial dose calibrators are generally adequate for measurement of the activities of β-emitters. However, in some cases, the measurement of 90Y can lead to errors reaching ±50%. For 169Er, with its much lower β-energy, the situation is even worse; the observed differences can be higher than 1 order of magnitude.
Measuring the activity of radiopharmaceuticals before patient injection is an essential monitoring procedure in nuclear medicine. Together with the producer’s surveillance of the radioactive isotope (test for radionuclide purity) and the labeling (test for radiochemical purity), it guarantees the safe use of radiopharmaceuticals in nuclear medicine. Activity measurements in nuclear medicine departments are part of good laboratory practice (1) and are being slowly integrated into national legislations (as in Switzerland, for instance) (2). Measurements usually are performed locally by use of a dose calibrator with the original vial provided by the producer if the whole preparation is to be injected. However, direct measurement with the injection syringe is preferred because it evaluates the solution actually injected, thus avoiding the problem of activity adsorbed to the vial wall. If, for a given radionuclide, several geometries can be measured in a dose calibrator, it becomes important for low-energy β-emitters to have geometry-specific calibration factors (3).
Measurements of pure β-emitters have been recognized as being problematic by the American Nuclear Regulatory Commission (4,5). For these radionuclides, the detection process does not involve photons directly emitted by the radioactive source. The β-radiation is absorbed in the source or in the vial and chamber walls and cannot reach the sensitive volume of the dose calibrator (except for a small fraction of β-energy typically of 2.5 MeV or higher) (6). Therefore, the measurements rely on bremsstrahlung production by the β-particles in the source. This situation leads to a very low sensitivity of the dose calibrator to the source activity, typically 2–3 orders of magnitude lower than is the case for γ-emitters.
Although measuring the activity of β-emitters is feasible (7), in daily practice, results of measurements of β-emitter activities with commercial dose calibrators are disappointing, because the activity readings are very different from those given by the suppliers of the radiopharmaceuticals. Three options can be adopted in such situations: one may trust the dose calibrator value, rely on the supplier’s activity and recalibrate the dose calibrator to check the stability of different batches, or accept as true the supplier’s indications and give up monitoring activity before injection.
The goal of this study was to investigate how such measurements are realized practically in nuclear medicine laboratories. We proceeded in 2 steps. First, a broad questionnaire was sent to all Swiss nuclear medicine laboratories asking which pure β-emitters were used and what kinds of routine measurements were realized. In a second step, a set of pure β-emitting sources were prepared, measured in a traceable manner, and sent to the laboratories to be measured.
MATERIALS AND METHODS
Preliminary Survey
Before the intercomparison exercise, a survey of the measuring methods of the Swiss nuclear medicine laboratories was performed. Questions were asked about the kinds of β-emitting radiopharmaceuticals used in the laboratory, the methods of activity monitoring measurements (geometric conditions) in place, and the calibration factors applied for the measurements. The opportunity was used to ask whether the laboratories were interested in participating in a subsequent intercomparison of β-emitter activity measurements.
Radioactive Sources
On the basis of the survey results, described below, 90Y and 169Er were selected as the pure β-emitters for the intercomparison.
90Y was chosen because it is widely used and its high maximal β-energy (2.3 MeV) leads to a relatively high instrument sensitivity that makes its measurement relatively easy.
The situation is the opposite for 169Er. Its low maximal β-energy (350 keV) makes it more difficult to measure because of the extremely low sensitivity of the dose calibrator. In this situation, the geometry of the instrument plays an important role given the strong attenuation of the low-energy bremsstrahlung produced (6).
Activity Determination
The radioactive sources were supplied by CIS Bio International. The chemical forms of the 90Y and 169Er batches were nitrite. This chemical form was chosen to provide real solutions that avoid partition problems during fractionation and dilution procedures. The source reference activities were measured by liquid scintillation with an accredited procedure traceable to national standards. These measurements were compatible with the activity values given by the suppliers.
Source Conditioning and Transport
The sources were conditioned in 15-mL glass vials with a 1.16-mm wall thickness. The variation in the wall thickness has an effect on the dose calibrator sensitivity. For 90Y and 169Er, this variation is typically lower than 2% and 5%, respectively (6). This effect was minimized further by selecting vials from the same batch.
All of the sources were delivered on the same day and were available at 7:00 am. The activities contained in each vial at 8:00 am were 71.0% ± 2% MBq for 90Y and 16.2% ± 2.5% MBq for 169Er (uncertainties are given with a coverage factor of 2). These activities are in the range of the measurements performed in the nuclear medicine laboratories.
Measurements Performed
The nuclear medicine laboratories were asked first to measure the sources in the original vials and then to measure the activity in the commonly used injection syringe when this corresponded to their regular practice. The suggested method consisted of filling the syringe with the volume used routinely and then measuring the activity in the original vial after it had been recapped. The activity difference between the 2 measurements was used to estimate the effective activity transferred to the syringe.
RESULTS
Preliminary Survey
Fifty nuclear medicine laboratories took part in the survey. Of the 50, 23 use pure β-emitters, 22 do not, and 5 did not reply. Of the 23 laboratories that use pure β-emitters, 4 did not take part in the subsequent intercomparison and 1 did not completely fill in the questionnaire.
Table 1 shows the number of laboratories using each of the radionuclides and the measuring methods. From the 22 completed questionnaires, it appeared that 16 laboratories do systematically measure the activities of pure β-emitters before injecting the patient. Three laboratories do it only for some of the nuclides, and 3 rely on the activity certified by the producer.
Measuring Methods Obtained from 22 Questionnaires
Intercomparison Results
Nineteen laboratories using at least 1 dose calibrator took part in the intercomparison, leading to a total of 27 instruments. Three types of dose calibrators are represented in this survey: Veenstra (Veenstra Intrumenten BV) with 18 instruments, Isomed (MED Nuklear-Medizintechnik) with 5 instruments, and Atomlab (Atomic Product Corp.) with 4 instruments. For analysis of the results, the measurements were distributed into 3 groups corresponding to the calibrator manufacturers.
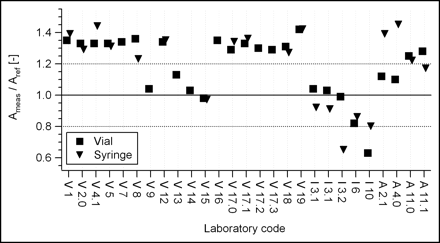
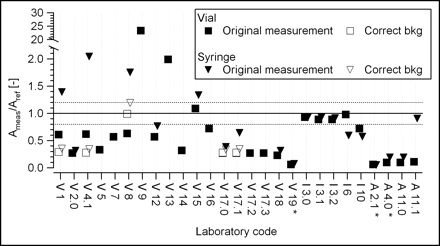
For each radionuclide, the ratios of the measured activity to the reference activity in vial geometry and, when available, in syringe geometry are presented. Of the 27 measurements performed in vial geometry, 20 were also performed in syringe geometry. The relative activities are displayed in Figure 1 for 90Y and in Figure 2 for 169Er. In the latter case, some measurements were repeated by us with corrected background subtraction.
Intercomparison results for 90Y. First letter in laboratory code represents instrument brand (V, Veenstra; I, Isomed; A, Atomlab). Laboratory codes without decimal points represent laboratories with only 1 instrument. Laboratory codes with decimal points represent laboratories with different instruments. Dotted lines represent an arbitrary tolerance of ±20%. Ameas/Aref [−] = ratio of measured activity to reference activity.
Intercomparison results for 169Er. Laboratory codes are as described in legend to Figure 1. Original measurement = measurements performed by laboratories according to their routine method. Correct bkg = measurements repeated by us with corrected background subtraction. Ameas/Aref [−] = ratio of measured activity to reference activity. *Measurements were performed with 32P scale.
For analysis of the results, it was assumed that, for β-emitters, a measurement with a deviation from the reference activity within 20% is still acceptable. The official Swiss requirement for γ-emitters is 10% (7).
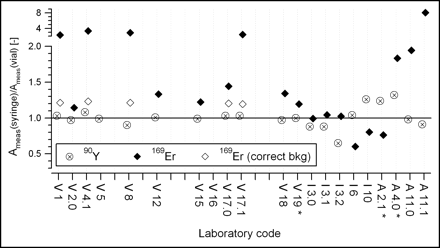
The ratios of the measured responses in both geometries are presented in Figure 3. The mean ratios of the measured activity to the reference activity for vial geometry and syringe geometry, along with their SDs, are given in Table 2. The number of measurements outside the ±20% tolerance is also indicated.
Ratio of activities measured in syringes and vials. Laboratory codes are as described in legend to Figure 1. Correct bkg = measurements repeated with special care concerning background subtraction. Ameas(syringe)/Aref(vial) [−] = ratio of measured activities in syringes to reference activities in vials. *Measurements were performed with 32P scale.
Results Distributed According to Radionuclide and Type of Geometry
DISCUSSION
Preliminary Survey
The high rate of responses to the questionnaire (45/50) and the high level of participation in the intercomparison exercise (19/23) are greatly satisfying; they confirm the interest of nuclear medicine laboratories in activity measurements for β-emitters.
The single most commonly used β-emitter is 90Y, which is used in 20 of 22 laboratories. It is followed by 186Re, 169Er, and 153Sm, which are used by more than one half of the laboratories. Finally, 32P and 89Sr are used by about one quarter of the laboratories.
The survey shows that both measuring conditions—in the original vial and in the syringe—are used and that this choice does not depend on the nuclides. This finding suggests that instrument manufacturers should provide calibration factors for both conditions when they differ.
We chose 90Y because of its widespread use and 169Er because of its frequent use and its low maximal β-energy, which is difficult to measure (8,9).
90Y Intercomparison
For the Veenstra dose calibrators, which account for two thirds of the instruments used by participating laboratories, the response in vial geometry can be divided into 2 groups: a first set of values at about 1.35 and a second set at about 1.0. Questioning of the manufacturer revealed that the instruments with a response near 1.0 had their calibration factors recently updated. It can only be regretted that the manufacturer did not implement this correction systematically to all dose calibrators in use. We also observed that the instrument response in syringe geometry was not significantly different from that in vial geometry. Therefore, geometry-dependent calibration factors do not seem to be justified for 90Y.
For the Isomed dose calibrators, 4 of 5 instruments were within the ±20% requirement for response precision in vial geometry. For the fifth instrument, a low value—also seen for erbium—was obtained. On the basis of the present measurements, it is unfortunately not possible to know the reason for this difference (poor calibration or poor measurement). Isomed calibration factors are specific to measuring conditions, and the user has to choose the relevant one from among a menu computed for a large number of vials and volumes. These factors were systematically used in this intercomparison. If these factors were correct, then the ratios of vial measurements and syringe measurements should be 1.0. However, the large variation observed brings into question the accuracy of these factors or their correct use.
Concerning the 4 Atomlab instruments, it was observed that the first 2 instruments (numbers 2.1 and 4.0) were of the type Atomlab-100. Their response in vial geometry was satisfactory, and their response in syringe geometry was relatively high. The other 2 chambers (instruments 11.0 and 11.1) were of the type Atomlab-200. Their response in vial geometry was slightly high, and their response in syringe geometry was lower and satisfied or was closer to the precision requirement.
169Er Intercomparison
A comparison of Figures 1 and 2 shows immediately that the situation for 169Er was far worse than that for 90Y.
For the Veenstra instruments, the ratios of vial measurements and syringe measurements were markedly varied. Because this result may have stemmed from incorrect calibration factors as well as measurement errors, the laboratories were asked to clarify the matter. It was revealed that the variation in the results could be explained by the fact that the background had not been subtracted correctly. Because of the low sensitivity of the instruments for this low-energy radionuclide, not subtracting the background correctly could lead to large errors. To test this hypothesis, we decided that new measurements should be performed in 4 laboratories taking into account the background current of the instruments. The results obtained were much less scattered (see corrected values in Figs. 2 and 3).
The measurements obtained in vial geometry indicated an underestimation by a factor of 3. As for 90Y, laboratory code V15, which has an updated version of the instrument software, indicates a correct response. The ratio of syringe geometry to vial geometry is about 1.2. This value justifies providing specific calibration factors for each measurement geometry.
For the Isomed instruments, 4 of 5 instruments gave satisfactory results in vial geometry. In syringe geometry, the results were acceptable for 3 instruments only.
The Atomlab-100 instruments (numbers 2.1 and 4.0) had no calibration factor for 169Er, and the 32P scale was used instead because 32P was the β-emitter available in the instrument software with the energy closest to that of 169Er. The other 2 Atomlab instruments (Atomlab-200) did have a calibration factor for 169Er. However, the results obtained with both types of instruments showed a marked underestimation of the activity, with a typical response of Ameas/Aref = 0.10. The large variation of the measurements in the syringe condition also probably is related to incorrect background subtraction.
CONCLUSION
This study shows that commercial dose calibrators are generally adequate for measurement of the activities of β-emitters. However, in some cases, the measurement of 90Y can lead to errors reaching ±50%. For 169Er, with its much lower β-energy, the situation is even worse; the observed differences can be higher than 1 order of magnitude.
These findings must be analyzed carefully because the observed discrepancies can be the result of incorrect calibration factors or measurement failures. For instance, in several cases, much better results were obtained with recent calibration factors provided by the instrument manufacturers. However, for 169Er, the repetition of some measurements by us showed that incorrect background subtraction had been performed.
This kind of intercomparison could have prompted the nuclear medicine laboratories to modify their calibration factors. However, this procedure is not recommended, because the correctness of the calibration factors should be the responsibility of the manufacturers. In order to address this problem, a meeting was organized with the representatives of the manufacturers, and the intercomparison results were presented there. The manufacturers reacted positively and planned to revise their calibration factors if they were not already in the process of doing so. They also planned to update the calibration factors for their customers and believed that the precision requirement of ±20% for β-emitter measurements was achievable.
Acknowledgments
The authors thank the leaders of the Swiss nuclear medicine laboratories for participating in the intercomparison and their staff for their commitment in performing the measurements. They are grateful to the representatives of the manufacturers for their collaboration. Finally, they thank Youcef Nedjadi for reviewing the editing of the manuscript.
Footnotes
For correspondence or reprints contact: François O. Bochud, Institut Universitaire de Radiophysique Appliquée, Grand-Pre 1, Lausanne, VD, Switzerland CH-1007.
E-mail: francois.bochud{at}chuv.ch