Abstract
A case of gross skeletal muscle uptake of 18F-FDG during PET is described. The clinical context of immunosuppression after heart and lung transplantation and the absence of any other known association make the former a likely etiologic factor.
The normal in vivo distribution of 18F-FDG during PET includes the brain, heart, kidneys, and urinary tract at 1 h after tracer injection. Skeletal muscle is known to show variable amounts of 18F-FDG uptake because it has a relatively high glucose metabolism. We describe a patient with gross 18F-FDG uptake involving multiple muscle groups and its possible link with immunosuppressive therapy.
CASE REPORT
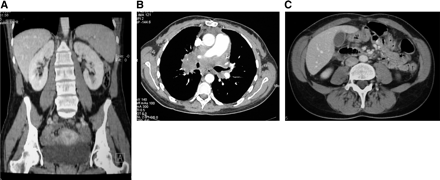
A 35-y-old woman with pyrexia of unknown origin was referred to the Nuclear Medicine Department for an 18F-FDG PET scan. She had undergone heart-lung transplantation for secondary pulmonary hypertension 4 mo previously. After transplantation she developed organizing pneumonia proven on transbronchial biopsies for which she required treatment with pulsed methyl prednisolone. Three months after surgery, she developed intermittent pyrexia with daily spikes of temperature up to 38.5°C. Her lung function was normal, and repeated bronchoscopy and transbronhial biopsies at that time revealed no evidence of rejection or persistent pneumonia. The chest radiograph and CT scan showed carinal and paratracheal lymphadenopathy (Fig. 1). On the clinical suspicion of lymphoproliferative disorder, she underwent a mediastinotomy and lymph node sampling, which revealed normal reactive lymph nodes with no evidence of malignancy. The whole-body PET scan demonstrated an irregular increase in tracer activity in the mediastinum consistent with the clinical suspicion of lymphoma (Fig. 2). In addition, markedly increased uptake was present in almost all major muscle groups, including those of the neck, thorax, pelvis, abdomen, and the extremities (Fig. 2). There were no obvious morphologic changes in these muscles on CT. The patient’s medication at the time of PET included the immunosuppressants tacrolimus and mycophenolate mofetil in addition to lansoprazole and pravastatin sodium. There was no history of hyperglycemia, insulin administration, or noticeable muscle activity before imaging. The plasma creatinine kinase (CK) remained normal before and after the PET study. A repeated bronchoscopy showed a large polypoid mass in the right upper lobe bronchus, which, on biopsy, confirmed high-grade B-cell posttransplantation lymphoproliferative disease.
Postintravenous contrast-media CT images acquired on 4-detector multislice CT unit. Muscles and corresponding fat planes are normal on all images. (A) Axial image of thorax shows extensive mediastinal lymphadenopathy. (B) Axial image of abdomen. (C) Coronal reconstruction of abdomen.
Attenuation-corrected whole-body emission images acquired (PET Advance; General Electric) 60 min after intravenous injection of 370 MBq (10 mCi) 18F-FDG. Orthogonal images show abnormal metabolic activity in mediastinum and extensive tracer uptake in muscles. Uptake is particularly prominent in neck, intercostals and abdominal muscles, diaphragm, and psoas.
DISCUSSION
18F-FDG PET is increasingly used in the effective management of oncologic patients (1). This imaging modality takes advantage of the overconsumption of glucose by tumor cells compared with normal tissues. However, 18F-FDG is not a tumor-specific substance and its accumulation in benign conditions may give rise to interpretive pitfalls (2,3). A detailed knowledge of these conditions as well as normal physiologic uptake at various sites is, therefore, important to avoid a mistaken diagnosis.
There have been several reports of exaggerated physiologic uptake of 18F-FDG involving different muscle groups during PET (4–7). The most commonly affected sites are the neck and upper thorax, and the uptake may be caused by muscle activity before, during, or after the radiotracer injection. This activity in turn may arise from talking, exercise, tension, or even shivering. In an attempt to reduce this so-called physiologic muscle uptake, some departments have used benzodiazepines before 18F-FDG administration with some success (8). Another cause of increased 18F-FDG muscle uptake is the administration of insulin in hyperglycemic patients (2). In the same way, hypoglycemia from any cause may enhance the muscle uptake. More recently, with the use of combined PET/CT units to accurately coregister the images, there has been concern that at least some of the presumed muscle uptake actually represents brown fat metabolism (9,10).
In our patient, the typical distribution of uptake and information from correlative CT leaves little doubt that the changes correspond to the muscles. Furthermore, the patient was so ill that she had not undergone any strenuous physical activity before scanning. She also had no history of shivering, convulsions, or insulin administration around the time of examination.
Inflammation is another recognized cause of 18F-FDG uptake in the muscle (11) and, given the gross 18F-FDG uptake, the possibility of myositis, primary or secondary (known side effect of pravastatin sodium), should also be considered. However, there were no clinical data to support this and the CK was normal.
The patient had immunosuppressive therapy with tacrolimus and mycophenolate mofetil after heart-lung transplantation. The former is a recognized cause of tremor and convulsions (12), but neither was evident at the time of the PET study. Mycophenolate mofetil is not associated with similar side effects. Nevertheless, both drugs are a known cause of altered glucose metabolism (12). It is, therefore, conceivable that the blood sugar might have dropped to a favorable level to augment muscle uptake. Alternatively, a previously unknown mechanism related to these drugs may have been the culprit.
The side-effects profile of lansoprazole does not indicate a causative role for the increased muscle uptake in this patient.
It could be argued that there was diffuse lymphomatous infiltration of the muscles. This would be exceedingly rare; one would expect at least some rise in serum CK and, as the CT images demonstrate, the muscles and surrounding fat planes appear absolutely normal.
In conclusion, although the intensity and extent of 18F-FDG uptake in this case are spectacular, the actual cause remains speculative. The burden of evidence, however, seems to be in favor of one or both immunosuppressive agents causing a metabolic disturbance, which in turn increases the 18F-FDG metabolism in all muscle groups. Such a mechanism would explain the global nature of the skeletal muscle uptake demonstrated in this patient compared with the published reports of selective uptake involving certain muscle groups.
Footnotes
For correspondence or reprints contact: Kottekkattu K. Balan, MD, Department of Nuclear Medicine, Box 170, Addenbrooke’s Hospital, Cambridge, CB2 2QQ, U.K.
E-mail: KBaladoc{at}aol.com