Abstract
Objective: Accurate activity measurements of glass conical v-vials are only possible if dose calibrator dial settings are experimentally determined for the specific vial and volume range over which the measurements of a particular radionuclide is to be made. V-vials are used to transport and store unit doses of radiopharmaceuticals containing high-energy beta-emitters, such as 186Re. We have determined the correct dose calibrator dial settings for measuring 186Re in 3-mL glass conical v-vials from 2 manufacturers. Methods: The 186Re solutions used were calibrated for radioactivity content at the National Institute of Standards and Technology (NIST) using liquid scintillation counting with 3H-standard efficiency with a maximum expanded (k = 2) uncertainty of 1.2% on the activity. Volumes of the solutions then were accurately dispensed into a set of v-vials from each of the 2 manufacturers and assayed in the dose calibrator maintained at NIST. Results: For filling volumes above 1 mL, the dose calibrator response was found to be constant for both of the vials studied, enabling a single dial setting to be used for each vial type. The expanded uncertainties on the activity from uncertainty in the dial setting in that volume range were 0.4%–0.7%. Variability in vial construction contributed another 0.2%–0.3% in the uncertainty in the activity determination. Conclusion: These studies indicate a strong volume dependence on the response of the dose calibrator and highlight the need for experimental verification of dose calibrator settings for nonstandard geometries.
One of the most popular instruments for performing assays of radioactivity in nuclear medicine continues to be the commercially available re-entrant ionization chamber, or dose calibrator. These instruments have the advantages of ease of use and acceptable measurement reproducibility. The accuracy of the device, however, depends on the correct calibration factor (dial setting) being applied for the nuclide and specific geometry under consideration. The dial settings recommended by most dose calibrator manufacturers are valid for only a single geometry. The dial settings are based on measurements made using sources in a standard geometry adopted by the National Institute of Standards and Technology (NIST). This geometry consists of 5 mL radioactive solution in a 5-mL flame-sealed glass ampoule with very thin walls and highly reproducible dimensions. In most applications, however, routine measurements generally are not made in this geometry. More typical sample configurations include various solution volumes contained in plastic or glass syringes, as well as glass dose vials, such as the widely used conical v-vial.
The conically shaped, interior-bottom vial has several advantages over a standard, flat-bottom vial, especially for removal of radioactive compounds or radiopharmaceuticals. The entire volume of solution can be withdrawn more easily and quickly from the conical-bottom vial by using a needle long enough to reach the bottom of the “V” portion of the vial. This reduces time of manipulation, which is important for reducing the hand dose to those handling the solutions. Furthermore, the vial can remain fully in the vial shield during this procedure, which also significantly decreases the radiation exposure. In the case of automated withdrawal systems, a conically shaped vial also improves the design of the device by reducing the total volume of solution needed for the procedure and by assuring proper transfer and filling of the syringe, pump, or other fluid pathway with the solution in the device.
One drawback to this type of vial, however, is that neither the glass thickness nor the solution volume varies linearly with the height of the vial. Because the response of these instruments is generally not constant along the length of the chamber (being lower at the extreme top and bottom of the chamber and gradually reaching a relatively flat maximum about 3 cm from the ends), a nonlinear dependence of the chamber response to the filling volume in the vial can be expected.
Rhenium-186 is being investigated for a wide variety of applications in nuclear medicine. At the present time, there is no recommended dial setting for measuring 186Re in a dose calibrator. The use of conical v-vials for shipping products containing 186Re solutions is being considered with the hope that dose calibrators can be used to perform quality control assays of the solutions in the v-vials by users. We undertook an investigation to determine empirically the dose calibrator settings for measuring 186Re solutions in both the standard NIST geometry (5-mL ampoule) and in conical v-vials with variable volumes.
MATERIALS AND METHODS
The volume dependencies of dose calibrator dial settings for 2 types of v-vials were studied in 2 separate experiments, each with a single v-vial type and separately calibrated 186Re solution. All dose calibrator measurements were made using a dose calibrator (CRC-12; Capintec, Inc., Ramsey, NJ) maintained at NIST.
Rhenium-186 was produced through the 185Re(n,γ)186Re reaction at the Missouri University Research Reactor (MURR), Columbia, MO. The activated rhenium metal powder is dissolved and oxidized in excess 30% (w/v) hydrogen peroxide in water and gently heated to form a perrhenic acid solution (HReO4). The excess hydrogen peroxide and water are removed by vacuum distillation, resulting in a yellow-orange colored, dry residue. This residue is then redissolved and diluted to the desired radioactivity concentration in either water or normal (0.9% w/v) saline. The pH of the solution is adjusted with either sodium hydroxide or sodium acetate to between 4.5 and 7.5. Vials of sodium 186Re perrhenate solution are dispensed, stoppered, and crimp-sealed and autoclaved for sterility.
Experiment 1: Ace Glass Vial Volume Dependence
The first vial investigated was a 3-mL conical v-vial (Ace Glass, Vineland, NJ) with physical dimensions, as supplied by the manufacturer, of 53 mm high × 25 mm outside diameter, and a wall thickness of approximately 4.2 mm. A stock solution, containing nominally 134 MBq Na[186Re]O4 in 5 mL 0.9% (w/v) saline, was shipped to NIST from Mallinckrodt, Inc (St. Louis, MO). This high massic activity prevented a direct measurement on the stock solution by liquid scintillation (LS) counting. Instead, a series of dilutions was necessary to prepare the various counting sources that would be used to quantify the activity of the solution. The first, labeled “Re-1-Dil-1,” was prepared from a portion of the stock solution using 0.9% (w/v) saline solution to give a dilution factor of 43.8439. The second, labeled “Re-1-Dil-2,” was prepared from a portion of solution Re-1-Dil-1 using 0.9% (w/v) saline to give a dilution factor of 3.0781 from Re-1-Dil-1, or a total dilution factor of 134.9559 from the stock solution.
All primary massic activity determinations (in units of MBq · g−1) were performed using 4πβ LS counting with the CIEMAT/NIST 3H-standard efficiency tracing method (1–3). This method is a protocol by which the LS counting efficiency for a cocktail of interest under known, varying quenching conditions is obtained by the efficiency of a closely matched (in terms of cocktail composition) standard. The counting data are used to establish a relationship between degree of cocktail quenching and the efficiency for 3H (tritium). The total efficiency, εtot, for 186Re at a particular quench value was calculated using the following equation:
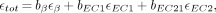 Eq. 1where bβ, bEC1, and bEC2 are the respective total beta and partial electron capture (EC) branching ratios and εβ, εEC1, and εEC2 are the calculated total beta detection efficiency and partial efficiencies for the respective EC branches. The efficiencies for the β− branches were calculated over the observed quenching range using a modified version of the program EFFY4, which is an updated version of EFFY2 (4).
Eq. 1where bβ, bEC1, and bEC2 are the respective total beta and partial electron capture (EC) branching ratios and εβ, εEC1, and εEC2 are the calculated total beta detection efficiency and partial efficiencies for the respective EC branches. The efficiencies for the β− branches were calculated over the observed quenching range using a modified version of the program EFFY4, which is an updated version of EFFY2 (4).
The input to the EFFY4 calculations consisted of the latest available nuclear data from the Evaluated Nuclear Structure Data File (ENSDF) (5). Two (allowed) decay branches were included in the calculations: the first has a decay energy of 1071.5 keV±1.3 keV and an intensity of 70.99%±0.12%; the second branch has a decay energy of 934.3 keV±1.3 keV and an intensity of 21.54%±0.14%. The remaining decay branches account for only 0.06% of the β− intensity and would have a lower efficiency relative to the other branches because they are either first forbidden or first forbidden-unique transitions.
The efficiencies of the EC branches were calculated with the program EMI (6) using the data in Table 1. For the EC branch to the excited state at 122.30 keV, the de-exciting gamma ray was included in the efficiency calculation.
Input Atomic and Nuclear Data for EMI Calculations of the Detection Efficiencies of Electron Capture (EC) Branches in the Decay of Rhenium-186*
LS cocktails were prepared for 186Re in glass vials, nominally 22-mL volume, fitted with polyethylene v-cone plastic caps. Four cocktails were prepared by adding 10 mL scintillation cocktail (Ultima Gold; Packard Instruments, Meridien, CT) to each LS vial, followed by the addition of 1 mL water. The degree of quenching was altered by the addition of between 0 drops and 12 drops of a 10% (by volume) solution of nitromethane in ethanol. To investigate the possible effects of LS cocktail composition on the efficiency tracing, an additional set of 4 vials was prepared by adding 10 mL Ultima Gold scintillation cocktail into each, followed by the addition of 1 mL saline solution and between 0 drops and 12 drops of the nitromethane solution. Nominally 0.025 g of solution from Re-1-Dil-2 was gravimetrically dispensed into each of the LS vials.
A total of 8 closely-matched LS cocktails containing 3H (as tritiated water) were prepared in a manner similar to the 186Re cocktails described above. Into each of the 8 vials, nominally 0.025 g of a diluted solution of NIST tritiated water standard SRM 4927E (7) were gravimetrically dispensed.
Four background blanks were prepared by the addition of 10 mL Ultima Gold scintillant to each of 4 LS vials. Into 2 of the vials was dispensed 1 mL water, while 1 mL of 0.9% (w/v) saline was added to the remaining 2 vials. A total of 12 drops of the nitromethane solution was added to 1 each of the water and saline cocktails. No quencher was added to the remaining 2 background blanks.
All cocktails were vigorously agitated for several minutes to ensure complete mixing and were sequentially counted for 10 cycles of 20 min per cocktail in each of 2 LS spectrometers over the course of 4 d. Counting rates for the first counting cycle in the first spectrometer were about 1.5 × 103 s−1 for the 186Re cocktails, thus minimizing the uncertainty in the deadtime correction.
Two point sources for counting in the NIST calibrated 4π NaI(Tl) detector system were gravimetrically prepared from solution Re-1-Dil-2 to enable confirmatory measurements to be performed by gamma-ray spectrometry. These sources were alternately counted over the course of 3 d for between 2,000 s and 10,000 s each, for a total of 3 repeated measurements on each source. An additional point source was prepared from solution Re-1-Dil-1 and was counted over the course of 2 d with calibrated high-purity germanium (HPGe) gamma-ray detectors to serve as a confirmatory measurement of the massic activity, as well as an analysis for radionuclidic impurities. The source was counted again after 6 d to analyze for long-lived inpurities. All counting data (LS and gamma-ray) were decay corrected to a common reference time of 1200 EST, September 16, 1998, using a half-life of 89.25 h (5).
Vials for dose calibrator measurements were prepared by gravimetric transfer of between (nominally) 0.12 g and 2.12 g of the original stock solution of 186Re into the Ace Glass v-vials. The vials were sealed using aluminum crimp-type seals with rubber/silicon septa. Because of the limited amount of solution available to perform the studies, filling the vials was accomplished in 2 runs. In the second run, the solution was recovered from the first set of vials and redistributed to another set of new vials. A total of 8 sources was prepared over the mass range 0.1 g to 2.1 g.
Each vial was placed in the NIST dose calibrator and the dial setting varied and the display output recorded, along with the time of the measurement. All data were decay corrected to the same reference time as was used in the activity measurements. Because all transfers of the stock solution were performed gravimetrically, the volume dependence of the dose calibrator settings was actually measured as a function of solution mass. The resulting mass dependence data can be converted to volume data by division using the density of the saline solution (nominally 1.006 g · mL−1).
Experiment 2: Wheaton Vial Volume Dependence, Wheaton and Ace Glass Vial Repeatability
The second type of vial that was studied was the 3-mL v-vial (Wheaton Science Products, Millville, NJ) that had a wall thickness of nominally 3.2 mm and outside dimensions of 46 mm high × 20 mm diameter, as specified by the manufacturer. In addition, several more Ace glass vials, similar to those used in Experiment 1 (but from a different manufacturing run) were used to study the variability in the dose calibrator measurements that were due to variations in the vials.
Approximately 15 mL of a stock solution containing about 1.02 GBq Na[186Re]O4 in 0.9% (w/v) saline was shipped to NIST from Mallinckrodt, Inc. As in Part I, a two-step dilution was performed using 0.9% (w/v) saline. The first solution, labeled Re-2-Dil-1 had a dilution factor of 19.3185, while the second, labeled Re-2-Dil-2, had a further dilution factor of 40.0021. This gave a total dilution factor of 772.7806 between Re-2-Dil-2 and the stock solution.
Liquid scintillation sources were prepared in a manner similar to that used in Experiment 1, with the following minor changes:
Five cocktails each for the 3H and 186Re were prepared instead of 8.
All sources contained 10 mL Ultima Gold scintillant and 1 mL saline solution.
The quench range was larger in that between 0 drops and 14 drops of the diluted nitromethane were added to cocktails.
Only 2 background blanks were prepared. One cocktail had no added dilute nitromethane, while the other had 14 drops of the quencher added to it.
All data were decay corrected to a common reference time of 1200 EST February 23, 1999, with the same half-life value used in Experiment 1.
A total of 7 vial sources was prepared in the Wheaton dose vials by the gravimetric addition of between (nominally) 0.1 g and 2.2 g of solution from the stock solution. Again, it was necessary to perform this part in 2 steps, recovering and redispensing the solution to use the limited solution volume. The vials were capped and measured in the dose calibrator exactly as was done in Experiment 1.
To study the variability between vials, a separate series of 4 vials each of both the Ace Glass and Wheaton types was prepared by the addition of 1.8 mL stock solution. To ensure the consistency of added 186Re, an automatic dispenser with a reproducibility of better than 0.1% (as quoted by the manufacturer) was used. The mass of added 186Re was then determined by mass difference of full and empty preweighed vials. The vials were capped and measured in the dose calibrator at dial settings of 450 × 10 and 475 × 10, and the times recorded. The adopted dial setting notation (e.g., 450 × 10) indicates that the instrument dial is set at the first number and the instrument readout value is multiplied by the second number. In addition, the chamber ionization current from the dose calibrator was read by means of a specially modified circuit that was added by the manufacturer at the request of NIST. To investigate possible variations between dose calibrators made by the same manufacturer, measurements were performed on a second dose calibrator (CRC-35R, Capintec, Inc., Ramsey, NJ) at the 475 × 10 dial setting. The data were decay corrected to the same reference time that was used in the activity measurements made on this particular solution.
RESULTS
Experiment 1
Analyses for possible photon-emitting radionuclidic impurities were performed on a point source prepared from the stock solution using HPGe gamma-ray spectrometry. No impurities were found to within a maximum of 0.75% of the emission rate of the 137.2-keV gamma ray in the decay of 186Re.
The massic activity of the stock solution used in this part of the study was measured to be 8.83 MBq · g−1 ± 0.11 MBq · g−1 as of the reference time, where the uncertainty is an expanded uncertainty using a coverage factor of k = 2. An accounting of the uncertainty components evaluated in the activity determination for this solution is given in Table 2. The confirmatory measurements made with both gamma-ray spectrometry methods agreed with the LS results to within their respective experimental uncertainties.
Uncertainty Components Evaluated in the Determination of the Massic Activity CA (in units of MBq · g−1) for Rhenium-186 Perrhenate Solution Used in Experiment 1
Because the sources used in this study were dispensed by mass instead of by volume, the results of the dose calibrator studies are given in terms of mass dependence. Conversion between mass and volume can be achieved by dividing the dispensed mass by the density (ρ = 1.006 g · mL−1).
The filling mass dependence of the Ace Glass v-vials was investigated for a total of 8 vial sources, covering the nominal range of mass 0.2 g to 2.12 g. A plot of these results is given in Figure 1. The data indicate no mass (volume) dependence for these vials over the range 0.96 g to 2.12 g, which effectively covers the region in the v-vial above the cone. The dial setting for this range that gives the correct activity is (447 ± 2) × 10. The cited expanded uncertainty in the dial setting over this range is based on the SD of the mean of the 4 values used in calculating the mean value. The expanded (k = 2) uncertainty on the activity arising from the use of this dial setting is 0.4%.
Volume dependence of dose calibrator (CRC-12) dial setting, DS, on filling mass, m, in 3-mL Ace Glass v-vials. The uncertainty bars correspond to an expanded uncertainty interval with a coverage factor of k = 2. The line through the data is the resulting fit using an equation of the form (DS)−1 = a + b/m, where a and b are fitting constants.
The mass (volume) dependence of the cone region of the v-vial can be approximated by the function:
 Eq. 2where DS is the calculated dial setting, and m is the mass (in grams) of solution in the vial. The associated expanded uncertainty on the dial setting calculated with Equation 2 is ±10 based on the average of the residuals of the fit to the data. The uncertainty in the activity obtained from using dial settings calculated with this equation was estimated by propagating the uncertainty in the dial setting through the fitted calibration curves for the vials, which had less than 1.0 g of sample in them. The average of these results leads to an expanded uncertainty in the activity of 3.9%, based on 4 determinations.
Eq. 2where DS is the calculated dial setting, and m is the mass (in grams) of solution in the vial. The associated expanded uncertainty on the dial setting calculated with Equation 2 is ±10 based on the average of the residuals of the fit to the data. The uncertainty in the activity obtained from using dial settings calculated with this equation was estimated by propagating the uncertainty in the dial setting through the fitted calibration curves for the vials, which had less than 1.0 g of sample in them. The average of these results leads to an expanded uncertainty in the activity of 3.9%, based on 4 determinations.
Experiment 2
The massic activity of the stock solution was measured to be 32.87 MBq · g−1 ± 0.18 MBq · g−1 as of the reference time, where the uncertainty were an expanded uncertainty using acoverage factor of k = 2. An accounting of the uncertainty components evaluated in this portion of the study is presented in Table 3.
Uncertainty Components Evaluated in the Determination of the Massic Activity CA (in units of MBq · g−1) for Rhenium-186 Perrhenate Solution Used in Experiment 2
The filling mass (volume) dependence of the Wheaton vials is presented in Figure 2. There was no mass dependence observed over the region 1.0 g to 2.2 g, which covers the region above the cone. The dial setting that gives the correct activity in this region is (462 ± 2) × 10, where the uncertainty is an expanded uncertainty estimated from the SD of the mean of the 4 dial settings for masses above 1.0 g. The expanded (k = 2) uncertainty on the activity arising from this dial setting is 0.7%.
Volume dependence of dose calibrator (CRC-12) dial setting, DS, on filling mass in 3-mL Wheaton v-vials. The uncertainty bars correspond to an expanded uncertainty interval with a coverage factor of k = 2. The line through the data is the resulting fit using an equation of the form (DS)−1 = a + b/m, where a and b are fitting constants.
The mass (volume) dependence of the cone region of the v-vial can be approximated by the function:
 Eq. 3where DS is the calculated dial setting, and m is the mass (in grams) of solution in the vial. The associated expanded uncertainty on the dial setting calculated with Equation 3 is ±5, again based on the average of the residuals of the fit. The uncertainty in the activity obtained from using dial settings calculated with this equation was estimated by propagating the uncertainty in the dial setting through the fitted calibration curves for the vials that had less than 1.0 g of sample in them. The average of these results leads to an expanded uncertainty in the activity of 1.6%, based on the average of 2 determinations.
Eq. 3where DS is the calculated dial setting, and m is the mass (in grams) of solution in the vial. The associated expanded uncertainty on the dial setting calculated with Equation 3 is ±5, again based on the average of the residuals of the fit. The uncertainty in the activity obtained from using dial settings calculated with this equation was estimated by propagating the uncertainty in the dial setting through the fitted calibration curves for the vials that had less than 1.0 g of sample in them. The average of these results leads to an expanded uncertainty in the activity of 1.6%, based on the average of 2 determinations.
The results of the evaluation of the uncertainty in the activity determination that was due to variability in the construction of the glass vials are presented in Table 4. Although the sample population was small, it appears that the measurement variability in the Wheaton vials is slightly less than that of the Ace Glass vials. To fully evaluate the uncertainty in the activity measurement made at the correct dial settings for each filling mass, the uncertainty related to choice of vial must be included in addition to that due to the determination of the dial settings themselves, as reported above.
Variability in Activity Determinations Due to Inconsistencies in Glass Vial Construction for the Ace Glass and Wheaton Conical V-Vials*
DISCUSSION
Volume-Dependence of Dose Calibrator Settings
It is not surprising that the dose calibrator dial settings vary as a function of the filling volume. It has been shown previously (8) that the response of the dose calibrator is not constant along the length of the chamber. The greatest and most constant response is found in the middle of the chamber, with a relatively sharp decline in the response starting about 1.5 cm above the bottom of the chamber. Because of this, solution sources with small volumes, in which the bulk of the activity lies in the bottom 2 cm of the chamber, will exhibit a lower response per unit activity than one in which the activity is closer to the center of the chamber. This effect is enhanced when the sources are counted in glass conical v-vials because the thickness of the glass vial varies as a function of height (and thus filling volume), causing nonlinear variability in the chamber response as a function of filling volume.
CONCLUSION
The volume dependence of the correct dial setting (DS) on filling mass, m, of solutions of 186Re perrhenate for a dose calibrator has been investigated for 3-mL glass conical v-vials made by 2 manufacturers. The results of the study indicate that the dose calibrator response per unit activity is constant for filling masses above 1 g.
The expanded uncertainties on the activity due to uncertainty in the dial setting for filling masses above 1 g were 0.4% for the Ace Glass vials and 0.7% for the Wheaton vials. The uncertainty due to variability in the vial construction contributes another 0.2%–0.3% in the activity determination uncertainty, depending on the vial type. Although the mass (m) dependence of the dial setting, DS, below 1 g in both the Ace Glass and Wheaton vials could be expressed by a function of the form DS = (a + b/m)−1, subtle differences in the glass thickness of each vial type resulted in different fitting constants a and b.
ACKNOWLEDGMENT
The authors thank Dr. Francis Schima of the NIST Radioactivity Group for performing the gamma-ray impurity analyses. Certain commercial equipment, instruments, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Footnotes
For correspondence or reprints contact: Brian E. Zimmerman, PhD, National Institute of Standards and Technology, 100 Bureau Dr., Stop 8462, Gaithersburg, MD 20899; Phone: 301-975-5191; E-mail: bez{at}nist.gov.









