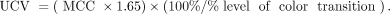RATIONALE
Alzheimer disease is associated with the accumulation of one or more misfolded proteins. The 2 primary proteins are amyloid-β and tau, which cause extracellular deposition of amyloid-β plaque and intracellular tau neurofibrillary tangles.
Although most patients with Alzheimer disease test positively for amyloid-β plaques, plaque deposition and cognitive impairment are poorly correlated. The consequent need for improved diagnostic tests and methods to monitor disease progression resulted in the development of 18F- flortaucipir. 18F-flortaucipir binds and labels tau neurofibrillary tangles.
CLINICAL INDICATION
Tau PET is indicated for estimation of the density and distribution of aggregated tau neurofibrillary tangles in adults with cognitive impairment being evaluated for Alzheimer disease.
CONTRAINDICATIONS
Patient agitation or inability to cooperate.
Pregnancy or breastfeeding (pregnancy must be excluded according to local institutional policy; if the patient is breastfeeding, appropriate radiation safety instructions should be provided).
A recent nuclear medicine study (radiopharmaceutical-dependent).
PATIENT PREPARATION/EDUCATION
The patient may eat and take medications as necessary before the procedure.
A focused history containing the following elements should be obtained:
○ Description of symptoms.
○ Neurologic symptoms and history.
○ Current medications.
○ Results of prior imaging studies, including CT, MRI, PET, and SPECT.
PROTOCOL/ACQUISITION INSTRUCTIONS
Obtain intravenous access via a short intravenous catheter (≤3.8 cm [1.5 in] to minimize adherence to the tubing).
Inject the radiopharmaceutical (Table 1) through the intravenous line as a bolus in a volume of 10 mL or less, and flush with 10 mL of normal saline. The patient may be seated or reclining when injected.
Follow the parameters detailed in Table 2.
Have the patient void before the image acquisition.
Position the patient supine on the imaging table, with arms down.
○ Use a head holder for proper positioning and to prevent motion.
○ Position the brain, including the cerebellum, in the field of view.
○ Avoid extreme neck extension or flexion.
Monitor the patient continuously during the procedure, with minimal interaction.
Radiopharmaceutical Identity, Dose, and Route of Administration
Acquisition Parameters: PET or PET/CT
IMAGE PROCESSING
Note that processing techniques vary by equipment manufacturer.
Reconstruct the images into transaxial slices with a 2- to 4-mm pixel size.
For iterative reconstruction, refer to the manufacturer’s recommendations for iterations, subsets, and smoothness.
Display the slices in the transverse, coronal, and sagittal planes.
Reorient the images to remove head tilt in the transverse and coronal planes.
Use a sagittal slice just off the midline to align the inferior frontal and inferior occipital poles in the horizontal plane.
Set the color scale:
○ Note that the goal is to identify and locate areas of 18F-flortaucipir activity in the neocortex that are more than background activity. Background activity equals 1.65 times the measured cerebellar average.
○ Draw a region of interest around the cerebellum in the transverse plane.
○ Select the plane to go through the cerebellum at the maximum cross-sectional area of the cerebellum.
○ Record the mean activity or cerebellar count (MCC).
○ Draw the region of interest on the grayscale images in the transverse plane.
○ Select a color scale for image display with a rapid transition between 2 distinct colors in the range of 25%–60% of maximum intensity.
○ Set the upper contrast value (UCV) of the color scale.
○ Set the visual threshold to 1.65 × MCC to match the rapid transition in the color scale:

Footnotes
↵* Reprinted from Farrell MB, Thomas KS, Mantel ES, Settle J. Quick-Reference Protocol Manual for Nuclear Medicine Technologists, 2nd edition. Society of Nuclear Medicine and Molecular Imaging–Technologist Section. 2024:141–143.
REFERENCES
- 1.
- 2.
- 3.