Abstract
The purpose of our study was to prospectively evaluate the striatal uptake of 123I-labeled N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane (FP-CIT) and the response to l-dopa therapy in patients with cerebrovascular disease (CVD) who develop clinical symptoms of vascular parkinsonism (VP). Methods: Twenty consecutive patients who developed VP in the course of CVD were prospectively enrolled in the study. All patients had CT evidence of CVD (17 patients had lacunar infarcts, 3 patients had territorial strokes). The clinical stage of the patients was assessed using the Hoehn and Yahr scale, and the severity of the symptoms was measured using the Unified Parkinson’s Disease Rating Scale score. Ten age-matched subjects were used as controls. SPECT was performed 180 min after injection of 185 MBq 123I-FP-CIT using a dual-head γ-camera. The ratio of the mean specific-to-nonspecific striatal binding for the entire striatum, caudate, and putamen was calculated in all patients and compared with that of controls. Putamen-to-caudate binding ratios were compared as well. The response to therapy was compared between patients with normal and abnormal 123I-FP-CIT binding. Results: No correlation was found between any of the clinical variables and response to therapy in patients with VP. Nine patients had normal striatal 123I-FP-CIT binding with no significant differences in striatal or subregional binding ratios compared with those of the controls. In contrast, 11 patients had significantly diminished striatal binding compared with that of controls (P < 0.001). Subanalyses showed significantly decreased binding in the caudate (P < 0.04 and P < 0.01 for the right and left caudate, respectively), diminished binding in the putamen (P < 0.04 and P < 0.01 for the right and left putamen, respectively), and a decreased putamen-to-caudate ratio on the right side (P < 0.001). The latter ratio was not significant on the left. Two of the 3 patients with territorial strokes had significantly diminished striatal 123I-FP-CIT binding in the hemisphere contralateral to the CT lesion. All 9 patients with normal scan findings had a poor response to l-dopa. Six of 11 patients with abnormal studies had no response to l-dopa, whereas 5 patients had a good response (P < 0.03). Conclusion: The diagnosis of VP cannot be accurately confirmed on the basis of clinical features alone because CVD may alter the typical presentation of PD. Functional imaging with 123I-FP-CIT is highly recommended in patients with CVD who develop symptoms of VP to confirm or exclude the existence of nigrostriatal dopaminergic degeneration. Identifying a subset of patients with reduced 123I-FP-CIT binding in the striatum is important for better treatment selection.
- dopamine transporters
- 123I-labeled N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane
- vascular parkinsonism
- Parkinson’s disease
- SPECT
The classical presentation of vascular parkinsonism (VP) is characterized by sudden onset, symmetric and rapid progression of motor symptoms, postural instability with shuffling gait, absence of tremor, and absent or poor response to dopamine substitution therapy, making it a clinical entity distinct from idiopathic Parkinson’s disease (PD) (1). The pathophysiology is poorly understood considering that only a minority of patients with basal ganglia vascular lesions develop parkinsonism (2), and neither the site nor the size of the lesions can predict the clinical presentation (3). The term “lower body parkinsonism” describing patients with gait disturbance but minimal upper limb involvement is also accepted as an indication of VP (4).
However, this presentation is variable in many patients with parkinsonism and vascular lesions (5–7), and data on the relationship between PD and stroke have been conflicting. For the clinician, the practical problem frequently is to determine whether a patient’s parkinsonism can be related to an identifiable cause other than a neurodegenerative process.
CT is often used to detect subcortical ischemia, basal ganglia lacunes, or infarcts (5,8) but it may not detect brain stem lesions (9). MRI (10–12) findings in VP are related to white matter hyperintensities with variable interpretation. Consequently, the diagnosis of VP cannot be reliably confirmed on the basis of clinical features or anatomic imaging modalities alone.
Dopamine transporters are localized on dopaminergic nerve endings and are lost in the process of degeneration in PD. They can be used as markers for the integrity or for the degree of loss of dopaminergic nerve endings. The cocaine derivative 123I-labeled N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane (FP-CIT) (DATScan; Amersham Health) binds with high affinity to dopamine reuptake sites in the striatum and can be used to visualize dopaminergic nerve terminals in vivo in the human brain. SPECT with 123I-FP-CIT is a sensitive marker of dopaminergic degeneration, and the degree of striatal binding reduction in PD correlates with disease severity (13,14). Dopamine transporter imaging offers the prospect of a quick, objective method to confirm or exclude presynaptic parkinsonism (PD) in inconclusive cases (15).
Whereas 123I-FP-CIT uptake is significantly reduced in the striatum of patients with PD, recent reports claim that dopamine transporters are usually normal in patients with VP or show only a slight diffuse reduction in the striatum with a normal putamen-to-caudate ratio (16). These findings may implicate mechanisms other than nigrostriatal dopamine loss that underlie the pathophysiology of VP and explain in part the lower response of VP patients to l-dopa.
The purpose of our study was to prospectively evaluate the striatal uptake of 123I-FP-CIT using SPECT and the response to l-dopa therapy in patients with cerebrovascular disease (CVD) who develop clinical symptoms of VP.
MATERIALS AND METHODS
Patients
Twenty consecutive patients (4 women, 16 men; mean age, 73 y; range, 60–83 y) who developed parkinsonism in the course of CVD were prospectively enrolled in the study. The study was approved by the local ethics committee. None of the VP patients had a prior history of PD and none used antiparkinsonian treatment at the time of study. The mean duration of disease was 22 mo (range, 3–72 mo).
All patients had CT evidence of CVD, 17 with lacunar strokes (10 of whom also had periventricular white matter changes) and 3 patients had territorial strokes. None of the patients had evidence of basal ganglia lesions. All had at least 2 risk factors for stroke according to Winikates and Jankovic (17). Cranial CT was used to exclude other causes of gait disorders, such as nonvascular focal lesions or hydrocephalus.
In addition, all patients were >60 y old (17–19) and had parkinsonism predominantly of the lower body (4,17). We excluded patients with cerebellar signs, marked autonomic failure, supranuclear gaze palsy, brain trauma, and any other neurologic signs unrelated to VP. Patients receiving neuroleptic therapy were also excluded. The clinical stage of the patients was assessed using the Hoehn and Yahr (Hoehn & Yahr) scale, and the severity of the symptoms was measured using the Unified Parkinson’s Disease Rating Scale (UPDRS) score.
For controls, we used the data of 10 aged-matched patients who had no indication of parkinsonism or CVD.
SPECT
All patients received potassium iodide (Lugol’s solution) orally to block thyroid uptake of free radioactive iodide. A dose of 185 MBq 123I-FP-CIT was injected intravenously and imaging was performed 3 h after injection. The SPECT study was performed using a dual-head γ-camera (Helix; Elscint) equipped with a low-energy, high-resolution collimator. A 20% window was centered on the 159-keV photopeak of 123I. One hundred twenty frames of 15 s were acquired using a circular rotation mode into a 128 × 128 image matrix. Transaxial, coronal, and sagittal slices 1 pixel thick were reconstructed using a third-order Metz filter set to 12-mm full width at half maximum. Attenuation correction was performed based on an automated ellipse determination with a constant linear attenuation coefficient of 0.11 cm−1.
SPECT Analysis
For analysis of striatal 123I-FP-CIT binding, 2 transaxial slices representing the most intense striatal binding were summed. Irregular regions of interest were constructed manually with the help of a brain atlas by the same examiner in areas corresponding to the right and left striatum, caudate, and putamen (Fig. 1). Irregular regions of interest were also drawn in areas corresponding to the medial occipital lobe on either side (700 mm2 each).
Illustration of region-of-interest placement on caudate and putamen.
The mean specific striatal binding was calculated by subtracting the mean occipital counts per pixel (nonspecific binding) from the mean counts per pixel in the whole striatum, caudate nucleus, and putamen (specific binding) and dividing the results by the mean counts per pixel in the occipital cortex. Putamen-to-caudate ratios were calculated as specific counts in the putamen divided by specific counts in the caudate nucleus. Low putamen/caudate ratios indicate that the loss of dopamine transporters is more severe in the putamen than in the caudate nucleus.
Statistical Analysis
Analysis of data was performed using SPSS statistical analysis software (SPSS Inc.). Ratios are presented as mean ± SD. Distributions of continuous variables were tested for normalcy using the Kolmogorov-Smirnov test. Ratios were compared simultaneously between patients and controls using 1-way ANOVA followed by the Bonferroni test for post hoc comparisons. Additionally, the t test for independent samples was used to detect differences in striatal 123I-FP-CIT binding ratios between patients and controls and between responders and nonresponders to therapy. The response to therapy (response vs. no response as assessed by the UPDRS score) was compared between patients with normal and abnormal scan results using the Fisher exact test. All tests were considered significant at P < 0.05.
RESULTS
Table 1 shows the clinical characteristics of patients with VP. No correlation was found between any of the clinical variables and response to therapy in patients with VP.
Clinical Characteristics of Patients with VP
123I-FP-CIT binding values in the whole striatum, the caudate, and the putamen are presented in Table 2. Additionally, the putamen-to-caudate ratio is shown for each patient. Nine patients had normal striatal 123I-FP-CIT binding with no significant differences in striatal or subregional binding ratios compared with those of controls (Table 3). In contrast, 11 patients had significantly diminished striatal binding compared with controls (P < 0.001 for right and left striatum). Subanalyses showed significantly diminished binding in the caudate (P < 0.04 and P < 0.01 for the right and left caudate, respectively), diminished binding in the putamen (P < 0.04 and P < 0.01 for the right and left putamen, respectively), and a significantly reduced putamen-to-caudate ratio on the right (P < 0.001). The latter ratio was not significant on the left. Two of 3 patients with territorial strokes had significantly diminished striatal 123I-FP-CIT binding in the hemisphere contralateral to the CT lesion.
Specific 123I-FP-CIT Uptake Observed in Striatum (S), Caudate (C), and Putamen (P) of Patients with VP
Specific 123I-FP-CIT Uptake (Mean ± SD) Observed in Striatum (S), Caudate (C), and Putamen (P) of Control Patients
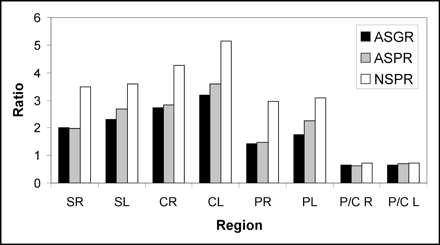
All 9 patients with normal scan findings had a poor response to l-dopa (Fig. 2). Six of 11 patients with abnormal studies had no response to l-dopa, whereas 5 patients had a good response (P < 0.03 compared with patients with normal scans). Figure 3 shows striatal 123I-FP-CIT binding in the patients with good response to therapy compared with a patient with a poor response.
123I-FP-CIT binding in different striatal regions in patients with good response and in patients with poor response to l-dopa therapy. ASGR = abnormal study with good response; ASPR = abnormal study with poor response; NSPR = normal study with poor response; SR = right striatum; SL = left striatum; CR = right caudate; CL = left caudate; PR = right putamen; PL = left putamen; P/C R = putamen-to-caudate ratio on right; P/C L = putamen-to-caudate ratio on left.
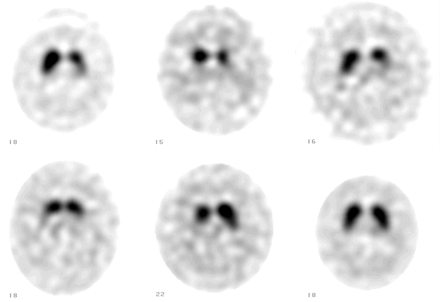
123I-FP-CIT SPECT of 5 patients with good response to therapy (upper row: left, middle, and right; and lower row: left and middle) and patient with poor response to therapy (lower row: right; patient 7).
DISCUSSION
Patients with VP are usually older, more likely to present with gait difficulty (especially freezing gate) rather than tremor (5,20–22), and less likely to respond to the use of levodopa compared with patients with PD (20,22). Pyramidal signs, dementia, or features of generalized arteriosclerosis are sometimes considered as further evidence for a vascular etiology in patients with parkinsonism (21). However, these atypical clinical features probably lack the sensitivity to detect the majority of the cases.
Clinical rating scales (Hoehn & Yahr and UPDRS scores) have a high sensitivity and specificity for the clinical diagnosis of PD (23). These rating scales, however, are more difficult to apply in patients with VP. Additionally, the clinical diagnosis is most difficult early in the disease when the signs and symptoms are quite subtle. Moreover, symptoms in PD become apparent only after a critical level of cell loss—the “symptom threshold” (24)—requiring a loss of approximately 80% of dopamine innervation.
Although VP has some characteristic clinical signs, our study does not support the concept of VP as a unique entity that can be clearly distinguished from PD. All of our patients had the typical clinical presentation of vascular parkinsonism by strict criteria, appearing during the course of CVD, and none had preexisting PD. Nonetheless, whereas 45% of the patients had intact dopamine transporters in the basal ganglia, a larger subset of patients (55%) had a significant reduction in the transporters, indicating the development of true nigrostriatal degeneration. We postulate that the latter group developed idiopathic PD, which was obscured by the underlying CVD, and therefore showed a significantly better response to levodopa therapy.
There is considerable difficulty in clarifying the relationship between vascular events and parkinsonism. Jellinger (25) found that, in a series of 617 consecutive patients with autopsy-proven PD, the total frequency of cerebrovascular lesions (lacunes, white matter lesions, old ischemic infarcts, and hemorrhages) was 44% in PD compared with 32.8% in controls, although the difference was not significant. van Zagten et al. (26) found one or more parkinsonian signs in 36% of stroke patients and at least 2 signs in 11% of patients. As in our study, parkinsonian signs were found more frequently in lacunar than in territorial stroke patients. It was postulated that both white matter lesions and lacunar stroke might damage the basal ganglia-thalamocortical circuit, resulting in parkinsonism. However, only 9% of patients with basal ganglia vascular lesions had parkinsonism, increasing to 25% if the patients had bilateral lesions (2).
Patients with VP evaluated by CT revealed evidence of either subcortical ischemia, basal ganglia lacunes, or infarcts (5,8). However, these lesions may coexist in otherwise typical idiopathic PD patients. In contrast to other studies on VP patients, of whom some had basal ganglia infarcts (16), our study is unique in selecting patients with no evidence of lacunar infarcts in this region. This excludes the possibility that abnormal 123I FP-CIT binding in some patients was due to basal ganglia ischemia.
The MRI description of white matter hyperintensity signals (11,27) is related to the increasing severity of ischemic tissue damage while some lesions (hyperintense periventricular regions) constitute areas of demyelination associated with subependymal gliosis, with varied interpretation. It is therefore conceivable that heterogeneity in the location, size, and extent of vascular lesions causes the diversity of clinical features seen in patients with VP (1) and might explain the overlap of striatal binding in patients with VP and idiopathic PD in some studies.
The cocaine derivative 123I-FP-CIT is a high-affinity radioligand for SPECT of dopamine transporters, which are situated in the membrane of dopaminergic neurons. Imaging of dopamine transporters is a sensitive marker to detect degeneration of the dopaminergic nigrostriatal pathway. Striatal 123I-FP-CIT binding is reduced in PD in proportion to disease severity (13,14,28). However, variability in uptake values suggests that factors other than nigrostriatal degeneration may contribute to disease severity. Studies with 6-18F-fluoro-l-dopa PET suggest that the disease process in PD first affects the posterior putamen, followed by the anterior putamen and the caudate nucleus (29). In contrast, most reports claim that striatal binding is preserved or only mildly reduced in VP, despite an overlap in some patients (16,30).
Although most studies emphasized the differences in striatal CIT binding between patients with VP and PD, diminished putamen-to-caudate ratios in some patients with VP and striatal binding overlap between the groups were not adequately explained (16). It therefore appears to us that the attribution of parkinsonian disorders merely to vascular lesions may be quite erroneous, ignoring the complex pathophysiology and consequences of cerebral ischemic injury.
Previous case studies reported hemiparkinsonism contralateral to territorial strokes, most likely related to injury of the striatum ipsilateral to the stroke. In contrast, we noted a marked reduction in dopamine transporters contralateral to a large hemispheric stroke in 2 patients. We therefore postulate that a direct ischemic insult to the striatum is not necessary for the development of parkinsonism. It is conceivable that crossed cerebral deafferentiation that commonly occurs in other brain insults (31,32) may induce striatal injury contralateral to a stroke in some patients, presumably in those with larger strokes.
The need for early detection of PD is highlighted by the substantial cell loss necessary before the manifestation of symptoms. Likewise, proper recognition of VP is important considering the possibilities for prevention of multiinfarct dementia and cardiovascular morbidity and mortality (17). Although it is impractical to screen all patients with CVD for PD, our data suggest that patients with CVD who show initial symptoms of parkinsonism should be evaluated with 123I-FP-CIT.
CONCLUSION
The diagnosis of VP cannot be accurately confirmed on the basis of clinical features alone since CVD may produce different forms of parkinsonism and alter the typical presentation of PD. Functional imaging with 123I-FP-CIT SPECT is highly recommended in patients with CVD who develop symptoms of VP to confirm or exclude the existence of nigrostriatal dopaminergic degeneration. Identifying a subset of patients with reduced 123I-FP-CIT binding in the striatum is important for better treatment selection.
Acknowledgments
We thank Dr. David L. Chamovitz for his editorial assistance.
Footnotes
Received Dec. 21, 2003; revision accepted May 21, 2004.
For correspondence or reprints contact: Mordechai Lorberboym, MD, Department of Nuclear Medicine, Edith Wolfson Medical Center, Holon, 58100, Israel.
E-mail: mvlorber@zahav.net.il