Visual Abstract
Abstract
Measurements of radionuclide uptake by the thyroid gland reflect its metabolic activity. Thyroid uptake is measured as a percentage of radioactivity retained by the gland at a specified time versus the activity administered to the patient; thus, uptake measurements must fall between 0% and 100%. Here, through a case study, we reviewed sources of error that can lead to uptake of more than 100%, and we describe a novel quality control (QC) indicator to improve the accuracy of uptake measurements in the clinic. Methods: Probe efficiency is determined as the ratio between the dose counts of the probe and the independent dose calibrator activity readings. The nominal probe efficiency value (M) was calculated as the mean of readings (n ≥ 20), and variance was characterized using the SD. Warning levels were set at M ± (1.96 × SD), and error levels were set at M ± (2.58 × SD). In subsequent routine clinical use, before a capsule is administered, the probe efficiency is calculated and compared with the warning and error limits. We derived M for 3 pairs of probe and dose calibrator devices using several doses and measured independently by several nuclear medicine technologists. Results: The recorded data indicated when technologists were made aware of the expected efficiency value, nominal efficiency was statistically different between our old device and the one that replaced it (P = 0.01), but coefficient of variation ([SD/M] × 100%) was not (P = 0.42). Using efficiency measurements acquired on the replacement device for the first 20 patients, we derived new QC values (M = 910, SD = 36). In 22 patients measured at our sister site, with the same device models but with the technologists unaware of the QC initiative, the derived QC values were an M of 1,025 and an SD of 116, demonstrating a significant difference between the nominal values of individual devices (P < 0.001). Furthermore, variability was significantly lower (P < 0.001) when QC was applied than when it was not. Conclusion: Adding probe efficiency as a QC indicator during thyroid uptake measurement is simple, can produce more precise clinical measurements, and can help mitigate operator and instrumentation errors.
Measurements of radionuclide uptake by the thyroid gland reflect its metabolic activity as well as the iodine handling and kinetics in the thyroid tissue. Such measurements show the fraction of radioactivity in the neck relative to that administered to the patient, with a predetermined time between measurement and administration (e.g., 24 h) (1). 131I- and 123I-sodium iodide, either in capsule or liquid form, is commonly used as the radiopharmaceutical for thyroid uptake determination. Clinical applications for this procedure include differentiation of hyperthyroidism associated with thyroid dysfunction (e.g., Graves disease or multinodular goiter) from other forms of thyrotoxicosis, such as subacute thyroiditis, and calculation of the activity of radioiodine (131I) to be administered for treatment (2). Because thyroid uptake is measured as a percentage of radioactivity retained by the gland at a specified time versus the activity administered to the patient (time = 0), uptake measurements must fall between 0% and 100%.
The basic procedure involves 4 steps (Fig. 1). First, the room background activity and administered activity are measured using a γ-counting probe, with the radioiodine dose positioned in a dedicated neck phantom. Duplicate measurements, including repositioning of the probe, are taken to avoid positioning errors, indicated by discrepant count rates between measurements. Highly discrepant dose counts (e.g., >10%) are investigated and addressed immediately to avoid propagation of errors. Second, the entire activity is administered orally to the patient. Third, at a predetermined time (e.g., at our institution, 24 h after administration), the patient returns for the uptake measurement, and the thyroid and patient background (thigh) counts are measured using the same γ-counting probe. Duplicate measurements, including repositioning of the probe, are taken to avoid positioning errors, indicated by discrepant count rates between measurements. Fourth, if the repeat counts match, the corresponding background measurements are subtracted and radionuclide decay correction is applied. The ratio of net uptake count rate to net administered count rate is the measured thyroid uptake, as shown in the following equation, in which the overbars represent the average of multiple repeat measurements.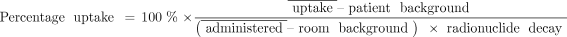 Eq. 1Modern probes are equipped with software to track the measurements and perform the thyroid uptake calculation automatically.
Eq. 1Modern probes are equipped with software to track the measurements and perform the thyroid uptake calculation automatically.
Thyroid uptake workflow diagram. At time 0, patient ingests dose of radioiodine that was measured at a prior time using thyroid probe. At 24 h after ingestion, patient returns to department for thyroid uptake measurement using same thyroid probe. Room and patient background measurements are performed with probe at time of dose and thyroid uptake measurements, respectively, for background subtraction before uptake ratio is calculated as percentage. Optional measurement of administered dose with dose calibrator can be used to calculate probe efficiency to be used for QC.
Because thyroid uptake relies on 2 measurements (steps 1 and 3) with few redundancies, quality assurance practices are essential to have confidence in the final reading. The counting technique is an important step, as the technique should include appropriate centering, distance, and positioning of the radiation-counting probe, thyroid phantom, and patient. The technique should be reproducible by technologists to ensure accuracy, and thus all counting should be performed in duplicate. Counting time should be set to a minimum of 60 s to ensure adequate photon-counting statistics (2). Finally, before the initial dose or uptake measurements, QC must be performed on the probe, including constancy to ensure that day-to-day counting efficiency is consistent.
Despite these quality initiatives, errors can and do occur. When thyroid uptake values exceed 100%, it is obvious that an error has occurred, but errors may not be detected if uptake values do not significantly deviate from the expected range (by other clinical indicators). Recently, a patient (patient 1) with known hyperthyroidism was referred to our clinic for thyroid uptake measurement and scanning before 131I therapy. The thyroid uptake was measured at 139% at 24 h. This event triggered an investigation by our local quality assurance committee, was discussed at our departmental mortality-and-morbidity rounds, and resulted in corrective actions.
Our clinic consisted of 2 sites, each performing 131I uptake measurements using Captus 3000 γ-probes (Capintec) that had reached the end of support by the manufacturer but were regularly maintained by our local biomedical engineering team. During the time in question, one of the machines was deemed unserviceable (site 1), and while a replacement was being procured, all patients were referred to our other site (site 2), which had a device of the same make and model. QC procedures consisted of routine maintenance and daily constancy tests performed according to manufacturer and professional society guidelines.
On the day that patient 1 was seen, 2 other patients were also referred to the clinic, and all 3 131I capsules (370 kBq) were measured in a single session in a CRC-55t dose calibrator (Capintec) and using the probe (2 duplicate measurements with room background subtraction). All 3 patients received their assigned capsule and returned for a 24-h uptake measurement, consisting of duplicate thyroid measurements and duplicate thigh measurements as patient background. The results are summarized in Table 1. The original results for patient 1 were clearly erroneous, exceeding 100% uptake, and thus the department physician and technologists immediately started an investigation while the patient was still present. Later that day, the reporting physician flagged the results of patients 2 and 3 as also being suspiciously high, on the basis of other clinical information. Thus, we suspected a technical error and investigated the following possible sources of error (2):
Thyroid Uptake Using 131I Capsules for 3 Patients
Operator error at thyroid measurement: During the 24-h visit of patient 1, uptake measured was confirmed by 2 independent measurements by 2 other technologists (3 measurements in total, agreeing within 5% of each other), including repeat measurements after removal of clothing. The patient returned for repeat uptake measurements at 48 and 192 h, and the measurements were 138% and 146%, respectively, indicating reproducible high uptake and trapping of the activity (i.e., no washout).
Operator error at radioiodine dose measurement: The process to measure radioiodine capsule counts was simulated with the technologist who had measured the 3 131I-capsules on the day in question. That technologist has over 20 y of experience and demonstrated proficiency in the procedure. No cognitive errors were identified. Furthermore, as expected, the count rates for all 3 capsules matched, as all 3 capsules were ordered to have the same activity (∼370 kBq [10 μCi]) and were from the same batch (Table 1). The 3 capsules were measured sequentially without repositioning of the probe or phantom between capsules.
Background measurements: The room and patient background should be measured near the time that the corresponding administered dose and thyroid measurements are done, respectively. Failure to do so increases the risk of inaccurate background readings that do not reflect changes in the environment in the ensuing time. The results of our case study (Table 1, patient 3) revealed a previously unidentified methodologic error in our clinical practice in which a single background measurement may be used for multiple doses measured hours apart. This practice is unlikely to be a significant source of error in this case, as the probe is housed in an area isolated from the main nuclear medicine department and with low patient traffic. Nevertheless, we have since revised our clinical protocol to state that all doses must be measured within 15 min of the corresponding background reading.
Instrumentation settings: The physicist of the Department of Nuclear Medicine was present for the 48-h assay and verified that the system settings were consistent with the department protocol.
Instrumentation QC: QC logs were reviewed for the probe and indicated consistent and in-range daily QC metrics over the week preceding and the week succeeding the dose administration. In our clinic, QC is performed according to manufacturer recommendations (3). Two reference sources (137Cs and 152Eu) are measured daily for energy calibration and precision, linearity, and efficiency constancy. Quarterly, we also perform χ2 testing of the counting performance of the system and test the minimal detectable dose.
Patient contamination: At 48 h, the physicist reviewed the emission spectrum from the thyroid assay and confirmed that the emission spectrum matched that of 131I, with no indication of contamination from other radioisotopes, and that low counts were present in the patient background reference region. Furthermore, radioactive contamination was ruled out by our radiation safety physicists by biologic assay of the patient and the patient’s spouse at 9 d after administration using a separate γ-counting probe. Thus, internal radioactive contamination was ruled out.
Correlation with prior measurement: The patient had a prior thyroid uptake measurement performed 32 mo earlier, measuring 51%.
Correlation with imaging: On the day of, and before, 131I administration, the patient was imaged with 99mTc-pertechnetate and a pinhole collimator. The images appeared to be visually similar between the 2 studies (Fig. 2); however, a quantitative comparison of 99mTc-pertechnetate uptake could not be conducted, as these images were acquired using different imaging devices (camera and collimator).
99mTc-pertechnetate uptake images of patient 1 at time of investigation (baseline) and 32 mo prior to that time. Image intensities were manually normalized to have similar contrast. Biodistribution is similar, contradicting large thyroid uptake change between time points.
Eventually, we identified a loose contact in the cable between the probe head and the data acquisition card of the device. How long this fault went unnoticed, and its implications on prior measurements, can only be conjectured. This fault was subsequently repaired by biomedical engineering staff and was followed by necessary calibration and QC, including χ2 testing.
To understand the potential implications regarding the 3 patients, we evaluated probe efficiency as the ratio of probe counts to dose calibrator readings using Equation 2 and as shown in Table 1. We compared these results with those of 14 previous patients in our clinic data (Table 2). Eq. 2Although we could not definitively determine the reason (e.g., probe physical configuration or instrumentation failure) for the change in geometric efficiency of the probe during capsule readings on that day, they appeared to be off by a fixed factor. We applied the ratio of efficiencies of the 2 cohorts to adjust the 24-h uptake using Equation 3, which aligned with the clinical histories of all 3 patients.
Eq. 2Although we could not definitively determine the reason (e.g., probe physical configuration or instrumentation failure) for the change in geometric efficiency of the probe during capsule readings on that day, they appeared to be off by a fixed factor. We applied the ratio of efficiencies of the 2 cohorts to adjust the 24-h uptake using Equation 3, which aligned with the clinical histories of all 3 patients. Eq. 3
Eq. 3
131I Capsule Activity, Probe Net Capsule Count, and Probe Efficiency Results at Clinic for Previous Patients
Nevertheless, we decided to replace this aging machine at site 2 with the same make and model as had been ordered for site 1. Furthermore, we began to implement new QC practices to mitigate similar risks in the future.
The purpose of the current study was to develop a QC method to mitigate errors when measuring radioiodine doses before their administration to the patient. We provide a detailed explanation of the method so that it can be applied by others. These methods include routine measurement of probe efficiency as part of the clinical workflow and comparison to prederived warning levels and error levels to initiate timely action by technologists, physicists, and biomedical engineering staff. We also explain how to derive the warning and error levels, and we provide a spreadsheet for data collection and calculations.
MATERIALS AND METHODS
As a clinical quality assurance study, this retrospective study was approved by the Institutional Research Ethics Board, and the requirement to obtain informed consent was waived.
After our recent upgrade, thyroid uptake measurements were again performed at our 2 sites, using identical thyroid uptake systems (Captus 4000e; Capintec) applying 60-s acquisitions with a 364 keV ± 10% photopeak energy window, and count rates were reported in units of counts per second (cps). To determine these devices’ counting efficiencies, the activities of several 131I-NaI capsules were measured in the respective site’s dose calibrator. The dose calibrators (Capintec CRC-25 and Capintec CRC-55t) were previously calibrated to a reference standard. Next, the 131I-NaI capsules were measured independently several times by several technologists in duplicate, and with room background count subtraction applied by the probes as would be performed clinically using the neck phantom holder.
Probe efficiencies were calculated for each probe measurement as in Equation 2. The nominal probe efficiency value (M) was calculated as the mean of all readings. Because count statistics follow a Poisson distribution and are sufficiently high (∼18,000), a gaussian distribution was assumed; therefore, efficiency variance was characterized using the SD of all measurements (4). Warning levels were set at M ± (1.96 × SD), and error levels were set at M ± (2.58 × SD) (Table 3), corresponding to an expected false-positive rate of 5% and 1% of capsule measurements, respectively (5). The ratio of the SD to the mean is referred to as the coefficient of variation (CV) and expressed as a percentage. Thus, warning and error levels can be expressed as a percentage of the nominal value or in absolute units (cps/MBq).
Example QC Limits for Probe Efficiency with 3 Levels
We determined the nominal probe efficiency, warning levels, and error levels for 3 devices: site 1, new device (Site1New); site 2, old device (Site2Old); and site 2, new device (Site2New). For Site1New, data were acquired from routine clinical worksheets in which no QC was performed on efficiency measurements. These data served as a baseline sample of the variability of the efficiency when QC is not performed. For Site2Old, technologists were explicitly instructed to perform multiple test measurements using multiple capsules on multiple days using the old probe (Captus 3000). In this case, the technologists were aware of the test being performed and paid attention to the expected probe count rates. These data were used to derive a baseline measure of nominal efficiency and its variability. For Site2New, data were collected from routine clinical worksheets in which QC was performed by the technologists on the efficiency measurements using warning and error limits derived from Site2Old (because the nominal values for these machines were similar in preliminary measurements).
In subsequent routine clinical use, before a capsule was administered, the probe efficiency was calculated in the same manner as during calibration and using Equation 2. The calculated efficiency was then compared with the warning and error limits posted at the corresponding site for the probe and dose calibrator used (Table 3). Directives on how to handle pass, warning, and error events were also posted and are detailed in the “Discussion” section.
The difference between nominal values for different devices was tested for statistical significance using a Student unpaired t test (6). Likewise, differences in percentage CV were evaluated using an F test. A P value of 0.05 was used as a cutoff for statistical significance.
RESULTS
Mean and percentage CV probe efficiencies are summarized in Table 4 for the 3 devices. Baseline efficiency estimates (Site2Old) consisted of 29 independent samples measured by 7 technologists, using 6 capsules on 6 separate days. The CV was 4%, resulting in the warning and error levels shown in Table 3. These exceeded the variability expected from count statistics alone (∼0.5%). Using these baseline QC limits as estimates for the new device at the same site (Site2New), 20 routine clinical patients were worked up. The recorded data indicated that nominal efficiency significantly differed between these 2 devices (P = 0.01) but percentage CV did not (P = 0.42); new QC limits were derived from these data for subsequent QC testing in the clinic.
Comparison of Probe Efficiencies and Their Variability for 3 Devices (and Practices)
The 22 participants who received a thyroid uptake measurement at site 1 (M = 1025, SD = 116), compared with the 20 participants at site 2 (M = 910, SD = 36), demonstrated a significant difference between individual machines (P < 0.001) with regard to nominal values, further justifying that specific QC limits were required for each device. Furthermore, in the absence of QC indicators at this site, variability was nearly 3 times greater (CV = 11% vs. 4%).
DISCUSSION
In this work, we set out to enhance the QC of thyroid uptake measurements by ensuring that 2 independent measurements of the dose administered to the patient are consistent: probe count rate and dose calibrator reported activity. We concluded that in our clinical practice, 4% CV was achievable, corresponding to approximately 8% and 10% warning and error limits, respectively, associated with 95% and 99% CIs, respectively. In other words, using these error and warning limits, we expect to experience false-warning and false-error limits for 1 of every 20 and 100 tests, respectively. These would trigger further investigation, which would be resolved before the dose is administered to the patient and therefore would lead to higher confidence in the final clinical results. It is possible that with further emphasis on QC, the variability (percentage CV) can be further decreased toward more precise thyroid uptake measurements.
We, like others previously (2), identified several possible sources of error in high thyroid uptake. We included this detailed description to guide readers in the event that they need to investigate a similar incident in their own clinic. Table 5 highlights a more complete list of potential sources of errors and means to mitigate their occurrence and propagation. However, for our enhanced QC, we focused on the preadministered dose measurement because it is a single point of failure that cannot be conclusively investigated after administration. Erroneous measurements of thyroid activity, on the other hand, can be investigated within several hours, assuming they are caught early enough.
Potential Sources of Error Leading to Erroneous Thyroid Uptake Measurements
An important finding in this work is that counting efficiencies vary between devices, even of the same make and model, and that nominal values and limits therefore must be determined for each pair of devices (probe and dose calibrator) unless explicit calibration is performed. An additional key finding is that QC testing of efficiency can reduce the variability in clinical practice, as demonstrated by the percentage CV between Site1New and Site2New, which were measured without and with QC, respectively. Obvious sources of variability that should be investigated when QC fails are transcription errors; probe, phantom, or source positioning; and changes in or malfunctioning of the instrumentation.
Radioactive Decay Correction
Probe efficiencies were calculated using Equation 2. This simple calculation ignores radioactive decay, as is acceptable if the dose calibrator and probe measurements are within 1 h of each other and if 131I is used, which has a physical half-life of 8 d (7). For shorter-lived isotopes or delays between dose calibrator and probe measurements, a decay correction may be required.
Clinical Application
To apply the proposed QC process in a clinical setting, we propose the following instructions, with tailoring to the clinic’s specific workflow and constraints. These instructions comprise 2 sequential steps: determination of QC limits and routine QC.
Determination of QC Limits
For each probe and dose calibrator pair, nominal efficiency values and tolerances must be determined by repeat measurement of sample capsules in a manner that represents the clinical workflow. Considerations include repeat measurements by different technologists on different days and using several doses that span the range of activities used in the clinic for the procedure. The exact methodology will vary depending on the number of technologists in the clinic, but we recommend 30 independent measurements, with 20 as a minimum to ensure adequate statistical power. Using the methods described in the “Materials and Methods” section, the nominal value and tolerances can be determined and posted in the laboratory for routine QC. An example of this QC table can be seen in Table 3. Other, clearly marked, variants—including the use of color, graphics, and accompanying instructions—should be considered in consultation with the technologist team to ensure optimal communication of new QC practices. A sample Microsoft Excel worksheet to derive QC limits from experimental data is provided as supplemental material (available at http://jnmt.snmjournals.org).
Routine QC
During clinical operations, each dose must be measured using a dose calibrator and then using the probe with the neck phantom. The ratio of probe counts to the dose calibrator reading must be calculated as in Equation 2, and the value must be compared with a table as demonstrated in Table 3.
Three possible scenarios arise. In the first, QC passes when the calculated efficiency is between the 2 warning levels. The clinical procedure should proceed as normally. In the second, there is a QC warning when the calculated efficiency is between a warning level and an error level (low or high). The work should be checked or repeated, including by an independent trained clinical staff member. If QC remains at a warning level, the clinical work should proceed if required (e.g., if there are workflow constraints or patient has traveled a great distance), but the physicist, quality manager, or biomedical engineering should be notified of the warning for further investigation. Also, the reporting physician should be notified. In the third scenario, a QC error occurs when calculated efficiency exceeds either lower or upper error levels. If the source of error cannot be identified and corrected, the capsule should not be administered to the patient until the physicist, quality manager, or biomedical engineering has been made aware of the error, has investigated the error, and has resolved the issue.
Liquid Iodine
In our clinic for diagnostic procedures, we currently use radioiodine in capsule form at a single dosage. Nevertheless, the same procedure may be applied to liquid form by preparing representative samples and measuring them both with the dose calibrator and with the probe. However, special accommodations may be necessary, including accounting for changes in the geometric efficiencies of the probe and dose calibrator, depending on the container and liquid volume (8).
Furthermore, one should ensure that a consistent efficiency factor is achieved across the entire range of activities used (e.g., if performing uptake measurement using therapeutic doses). This assurance requires that both systems operate in a linear range across the full range of activities and that probe dead times remain below approximately 2%. If greater dead-time factors must be accommodated, a more complicated, activity-dependent efficiency curve may be required.
Strengths and Limitations
These additional QC practices can be implemented in a routine clinical setting with little impact on workflow. This QC provides an extra layer of assurance to boost confidence in the validity of the thyroid uptake results. However, it is important to appreciate a remaining limitation: if the probe efficiency varies between the day of capsule measurement and the day of patient uptake (e.g., 24 h later), erroneous thyroid uptake measurements may still result and go undetected. Therefore, daily QC testing of the probe, including constancy, is still essential to achieve high-quality thyroid uptake measurements.
In this work, we do not report on QC outcomes in our clinic after full implementation of this QC procedure. There have been too few data for meaningful analysis to date (14 at site 1 and 10 at site 2), although none of the data have resulted in warning or error events that triggered investigations by our QC team.
At our institution, thyroid uptake is typically measured at 24 h after oral administration of an 131I capsule. Ideally, the thyroid uptake should be measured at multiple time points to accurately characterize the biologic process, including identification of patients with a rapid turnover time with higher uptake at 4 or 6 h. Acquiring data at these additional time points may also benefit QC and investigation of anomalous measurements. Likewise, 99mTc-pertechnetate count rate measurements from the imaging studies may have aided investigation in the case study, but quantitative comparison was hampered by the use of different devices between time points. Use of standardized imaging equipment and protocols within the clinic is therefore advised whenever possible.
CONCLUSION
Thyroid uptake measurements can be prone to operator and instrumentation errors that cannot be detected without QC testing. The ratio between dose counts of the probe relative to independent dose calibrator activity readings is a simple QC indicator that can readily be applied in a clinic to reduce such errors.
DISCLOSURE
Ran Klein receives revenue shares from the sale of rubidium generators from Jubilant-DraxImage and from the sale of myocardial flow quantification software from Invia Medical Solutions. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can a thyroid probe efficiency test serve as a QC measure toward accurate thyroid uptake measurements?
PERTINENT FINDINGS: Before the radioiodine dose is administered to a patient, the dose can be used to test the thyroid probe–reported count rate against the dose calibrator--reported activity to identify errors exceeding approximately 10%. Many other potential sources of error can facilitate investigation of anomalous thyroid uptake measurements.
IMPLICATIONS FOR PATIENT CARE: This quality assurance measure can easily be implemented in a nuclear medicine clinic to improve quality and confidence in thyroid uptake measurements.
ACKNOWLEDGMENTS
We thank the staff of the Department of Medicine at The Ottawa Hospital for their collaboration in investigating this case and for their ongoing support in improving quality in our department.
Footnotes
Published online Dec. 6, 2021.
REFERENCES
- Received for publication June 16, 2021.
- Revision received October 8, 2021.