Abstract
The role of nuclear medicine diagnostic bone scanning is well established in prostate cancer. This case provides an insight into the specific role that bone scanning plays in monitoring response to hormone therapy and an example of significant global skeletal response. The case highlights the remarkable efficacy of timely hormone therapy in high-grade prostate cancer with widespread bony metastasis. In addition, the range of hormone therapy currently available for clinical application in the management of metastatic prostate cancer is detailed. Finally, the case represents an incidental diagnosis of prostate cancer after evaluation of nonspecific symptoms.
We present a case study that provides an insight into the specific role that bone scanning plays in monitoring response to hormone therapy. The case highlights the remarkable efficacy of timely hormone therapy in high-grade prostate cancer with widespread bony metastasis.
CASE REPORT
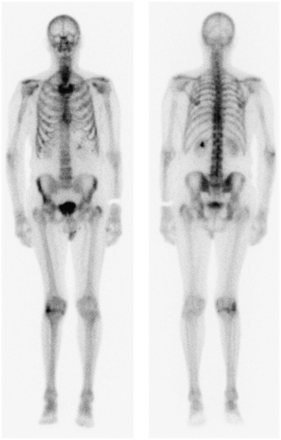
An 83-y-old man presented for evaluation of reported weight loss and constipation. These symptoms were initially assessed by CT of the chest, abdomen, and pelvis. The findings were unremarkable, although a left renal staghorn calculus was noted. Despite no previous history of neoplasia, extensive sclerotic lesions were noted throughout the axial skeleton. This finding, combined with the observation of an irregular prostate contour (without enlargement), raised suspicion of previously undiagnosed widespread metastatic prostate carcinoma. Subsequent follow-up revealed a prostate-specific antigen (PSA) level of 823 μg/L (normal, <4 μg/L) and bone scan findings consistent with widespread skeletal metastases (Fig. 1). Subsequent prostate biopsy confirmed aggressive prostate carcinoma with a Gleason score of 9 (1). The patient commenced hormone therapy using bicalutamide (150 mg; Cosudex [AstraZeneca]) daily (oral tablet) and leuprorelin (30 mg; Lucrin [AbbVie]) depot (intramuscular injection) every 4 mo. The medications were well tolerated. At week 4, goserelin (Zoladex; AstraZeneca), a 10.8-mg subcutaneous implant (1 dose at 3 monthly intervals), was added to the patient’s antitumor therapeutic armamentarium.
Baseline nuclear medicine bone scan demonstrating widespread metastatic disease associated with prostate carcinoma.
At 5 mo after the commencement of therapy, the PSA level had normalized to 0.39 ug/L. It dropped further to 0.11 μg/L at 10 mo, corresponding to scintigraphic resolution of bony metastatic changes on the follow-up nuclear medicine bone scan (Fig. 2).
Follow-up nuclear medicine bone scan demonstrating remission of metastatic disease after hormone ablation therapy.
DISCUSSION
Hormone Therapy
The approach taken in the treatment of prostate cancer will depend on several factors, including the clinical presentation, Gleason score, and PSA (2). For patients with metastatic spread of prostate cancer, such as the patient in this case, the treatment of choice is androgen-deprivation therapy (ADT) (2,3). ADT has been reported to lead to remission in as many as 80% of patients with advanced prostate carcinoma (3,4). ADT is used in advanced prostate cancer to relieve pain, prevent pathologic fractures, and prevent neurologic complications (4). Typically, androgen deprivation is achieved through 3 mechanisms in prostate carcinoma patients, gonadotrophin-releasing hormone (GnRH) agonists, antiandrogens, and GnRH antagonists.
GnRH agonists (goserelin, leuprorelin, and triptorelin) are considered the leading approach to ADT in prostate carcinoma (5). GnRH is released in pulses from the hypothalamus to stimulate pituitary release of luteinizing hormone, which, in turn, stimulates the secretion of testosterone in the testes (5,6). GnRH agonists decrease testosterone levels by overstimulation, downregulation, and eventually receptor desensitization (5–7). There is, however, an initial flare or surge in testosterone production before suppression is effected (5). Unfortunately, testosterone suppression also increases the risk of osteoporosis and fracture (loss of bone mineral density), diabetes (altered glucose tolerance), and cardiovascular disease (7). Some of the more common adverse effects of ADT include weight gain, erectile dysfunction, decreased libido, gynecomastia, insomnia, sweats or chills, and gastrointestinal disturbances (7).
Antiandrogens (bicalutamide, flutamide, and nilutamide) are nonsteroidal inhibitors of androgen receptors (6,7). The advantage of this class of ADT medications is that testosterone levels are not suppressed, and thus the associated risks discussed above are largely avoided (6,7). That is, antiandrogens inhibit conversion of testosterone to dihydrotestosterine (metabolite) and prevent both from binding to the androgen receptor, without suppressing serum testosterone levels. The flare in serum testosterone associated with GnRH agonists means that they should be avoided as monotherapy in patients with severe pain or neurologic problems. Antiandrogens can be used as adjunctive therapy to block the flare (4).
GnRH antagonists (degarelix) have only recently received attention in the literature. GnRH agonists can cause an early increase in testosterone levels which, in turn, can promote tumor spread and worsen symptoms (2). GnRH antagonists can be used before the GnRH agonist therapy to block testosterone increases (2,7). GnRH antagonists can also be used as an alternative approach to androgen deprivation by inhibiting gonadotrophin production, leading to a reduction in the synthesis of androgens in the testes (7).
Bone Scan
ADT has been shown to result in remission in as many as 80% of patients, which is reflected in a median period of 12–33 mo of progression-free survival (4,6). That is, ADT prevents the progression of metastatic spread. Typically the nuclear medicine bone scan will demonstrate partial improvement or stabilization (nonprogression) of metastatic disease in response to ADT, but it is a rare occurrence for the bone scan to revert to normal (4). This observation reflects the fact that ADT rarely provides an overall cure and the likelihood of cure is inversely proportional to the extent of disease at the time of commencement of ADT. Another reason for the infrequency of a completely normal follow-up bone scan is that remodeling of bone in response to healing will mimic metastatic progression. The reversion to normal in this case is remarkable given the advanced stage of disease (Gleason score of 9) and extent of metastatic disease at the commencement of ADT.
After the progression-free survival period, there will be transformation of the disease to an androgen-independent phenotype that will not respond to ADT (6). At this point, alternative therapy (e.g., docetaxel) may be indicated. The mean patient survival (from commencement of ADT) is 23–37 mo (6). Androgen-independent disease is marked by disease progression despite testosterone levels below castrate levels (<50 ng/mL).
CONCLUSION
The bone scan provides a valuable tool in monitoring response to ADT because, independently of PSA levels and anatomic imaging (e.g., radiography and CT), this modality demonstrates metabolic bone changes. This case demonstrates an unusually dramatic improvement in bone scan appearance after hormone therapy in prostate cancer.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We acknowledge urologist Dr. Steven Sowter for providing information to aid the formulation of the case study.
Footnotes
Published online Feb. 12, 2013.
REFERENCES
- Received for publication December 2, 2012.
- Accepted for publication January 28, 2013.