Abstract
99mTc-Mercaptoacetyltriglycine is used for dynamic renal imaging, and the summary of product characteristics (SPC) for the European formulation specifies a shelf life of 1 or 4 h, depending on the reconstitution volume of the kit. To minimize the time required to test the radiochemical purity, a simplified quality control method was developed. Methods: To satisfy the recommendations of the International Commission on Harmonisation, results obtained with the methods described in the European and American SPCs were compared with those obtained with the simplified method. Further validation of the new method was performed by comparison with the standard 2-strip thin-layer chromatographic method as well as tests for linearity and limits of detection and quantification. Results: The simplified method provided results comparable to those provided by the registered SPC methods but was more rapid to perform and used smaller volumes of solvents. Conclusion: The simplified method is a reasonable alternative to the registered SPC methods.
Technetium-99m-mercaptoacetyltriglycine (99mTc-MAG3) is a radiopharmaceutical used in dynamic renal imaging. It is prepared by adding sodium pertechnetate to a sterile lyophilized kit and heating for 10 min in a boiling water bath. The current summary of product characteristics (SPC) distributed with the European 99mTc-MAG3 kit describes 2 methods for testing the radiochemical purity (RCP) of the product, high-pressure liquid chromatography (HPLC) and the use of a solid-phase extraction (SPE) cartridge. Although HPLC is the more accurate method, the SPE method is more convenient for routine use and has been shown to provide equivalent results (1). The SPC distributed with the American 99mTc-MAG3 kit describes a slightly different SPE method for determination of the RCP. The 2 methods are compared in Table 1, where it can be seen that the European method is more complicated than the American method because of the requirement for phosphate buffer and 2 different concentrations of ethanol.
Comparison of SPE Methods for Determination of RCP of 99mTc-MAG3
Both the European and the American SPE methods for RCP testing require the slow elution of comparatively large volumes of solvents. Vinberg has shown that the elution rate of the SPE method is important (2); indeed, the elution rate is crucial if reliable results are to be obtained, with slower elution rates yielding higher RCP values. An elution rate of 1.5 mL/min was found to be the maximum limit, although recent work suggested that slow elution rates are not critical (3). It is therefore proposed that the European and American SPE methods may not be the best use of limited equipment and time in radiopharmacies and that a new, simpler, and quicker method should be developed and validated. Accordingly, we have developed a simplified method that addresses some of these limitations. The new method is quicker and uses fewer solvents. In addition, the method helps reduce the possibility of spurious results being obtained because of an overly enthusiastic operator. The method is summarized in Table 2.
Description of Simplified Method
The ICH Q2B regulations of the International Commission on Harmonisation (ICH) describe the requirements of a new test method that need to be satisfied for the method to be accepted as valid (4). Any new analytic method needs to be validated against the current registered methods to ensure compliance with the ICH Q2B regulations. Accordingly, our new method was validated against the methods specified in the European and American SPCs.
MATERIALS AND METHODS
All radiopharmaceutical kits (Technescan MAG3, European formulation; Tyco Healthcare) were prepared with 99mTc-pertechnetate eluted from a 99Mo–99mTc generator (Drytec; Amersham Health) and heated for 10 min in a shielded boiling water bath. The kits were prepared in accordance with the SPC, except in circumstances when larger quantities of 99mTc (up to 2,000 MBq) were added. All activities were measured with a Capintec CRC 15R dose calibrator.
Solid-Phase Extraction Cartridge Methods
The SPE cartridge used was the C18 Sep Pak (P/N 20515; Waters). The general procedure was as follows.
Prewet (“activate”) the cartridge with ethanol and then prepare the cartridge with preparation solvent.
Place a drop of the radiopharmaceutical in the inlet of the cartridge.
Elute sequentially with quantities of solution A and collect in a tube.
Elute sequentially with quantities of solution B and collect in a second tube.
Place the cartridge in a third tube for measurement of residual activity.
Measure the activity in each tube in the dose calibrator.
Calculate the RCP as the activity in fraction B divided by the total activity in all fractions.
To determine the profile of elution from the cartridge, solution B was collected in four 2.5-mL fractions.
Thin-Layer Chromatographic Method
The standard instant thin-layer chromatographic (ITLC) method developed by Chen et al. (5) was compared with the new method. The method of Chen et al. (5) involves the use of 2 strips of instant thin-layer silica gel (ITLC-SG; Pall Gelman) as the stationary phase. The mobile phase for the first strip is freshly prepared ethyl acetate:butanone (3:2). The strip is cut at the midpoint, and the activity at the solvent front represents the hydrophilic impurities, primarily free pertechnetate and 99mTc-tartrate. The mobile phase for the second strip is 50% acetonitrile. The strip is cut at the quarter point, and the activity at the origin represents the reduced hydrolyzed technetium (colloid).
Statistical analysis between groups was carried out by use of ANOVA with a significance level of P < 0.05.
Specificity Experiments
99mTc-MAG3 was prepared at 2,000 MBq in a 4-mL quantity, and the RCP was determined with the 3 different SPE methods. The high-activity preparation already was established as being stable from previous work in this laboratory and was used to reduce error attributable to the inability of the dose calibrator to read low activities. This procedure would determine whether the simplified method showed the same specificity for 99mTc-MAG3 and for hydrophilic and lipophilic impurities as the American and European methods.
Linearity Experiments
Known amounts of free pertechnetate were added to 99mTc-MAG3 preparations after boiling to produce various purities of 99mTc-MAG3. The RCP values of these solutions were tested with the simplified method to show its linearity over the expected working range of an analytic method.
Accuracy and Precision
The simplified method was evaluated for accuracy and precision by comparison against the standard ITLC method developed by Chen et al. (5) with impure mixtures prepared for linearity testing.
Limits of Quantification and Limits of Detection
Tests of limits of quantification and limits of detection are not required, as RCP values of less than 90% are not used in clinical studies, and the linearity response showed that RCP values of as low as 25% are detected and quantified.
Robustness
Robustness was evaluated by allowing other staff members to use the method and then determining the consistency of the results.
RESULTS
The ICH Q2B regulations state that validation of a new, simplified method needs to demonstrate the following: specificity, linearity, accuracy, precision, detection limit, quantitation limit, and robustness (4). Each was assessed individually as described below.
Specificity Experiments
Table 3 shows the average results of 6 individual experiments for each method along with the SDs. Two-factor ANOVA revealed no significant differences among the methods (n = 18, F17 = 2.67, P = 0.118, not significant).
Comparison of Distribution of Radioactivity Determined by 3 SPE Methods
Figure 1 shows that the amount of complex in the last 2 fractions was minimal and that the bulk was found in the first 2 fractions. Thus, the amount of solvent required to elute 99mTc-MAG3 was not as large as the SPCs suggest. The simplified method compared very well with the European and American SPE methods for determining the RCP.
Distribution of 99mTc-MAG3 in 2.5-mL fractions of solution B. Error bars indicate SDs.
Linearity Experiments
The measured RCP was plotted against the nominal RCP and analyzed by linear regression with the method of least squares. In 6 independent experiments, the r2 values were 0.9917, 0.9868, 0.9990, 0.9926, 0.9926, and 0.9966. The results showed very good correlation with each other (mean, 0.9932; SD, 0.0039). These data showed that the linearity criteria stated in the ICH guidelines were met.
Accuracy and Precision
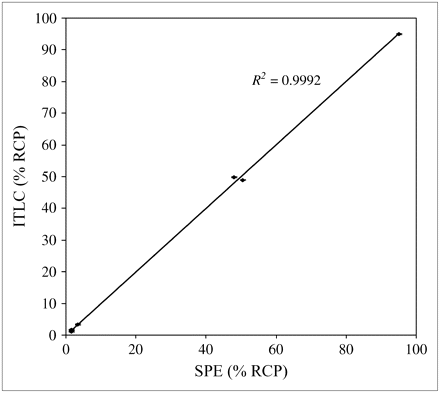
As shown in Figure 2, there was excellent agreement between the 2 methods. Single-factor ANOVA revealed no significant difference (n = 18, F17 = 4.67 × 10−9, P = 1.0, not significant). The slope of the regression line was 1.0025, and the intercept was 0.0863, indicating no systematic difference between the 2 methods and confirming the accuracy of the new method. The coefficients of variation for triplicate measurements were 0.06% for the new method and 0.18% for the ITLC method, indicating similar degrees of precision of the 2 methods.
Relationship between ITLC method and simplified SPE method.
Limits of Quantification and Limits of Detection
The dose calibrator can detect activities as low as 0.05 MBq, providing assurance that the new method can be used to detect low traces of activity and thus RCP.
Robustness
The results of further experiments performed by other radiopharmacy staff members indicated that the new method was robust.
DISCUSSION
Preparation of 99mTc-MAG3 involves adding 99mTc-pertechnetate to a kit containing a lyophilized mixture of S-benzoylmercaptoacetyltriglycine (betiatide, active ingredient precursor), sodium tartrate (weak chelating agent), and stannous chloride (reductant) (the American formulation also contains lactose as a bulking agent to aid in lyophilization) and heating for 10 min in a boiling water bath. Upon the addition of pertechnetate to the kit, a weak intermediate, 99mTc-tartrate, forms and is transchelated to MAG3 as the S-benzoyl protecting group is cleaved during heating.
The specification of a minimum acceptable RCP varies with different sources. The European Pharmacopoeia specifies 94% bound, determined by a combination of HPLC and thin-layer chromatography (for the measurement of colloid). The European SPC specifies a limit of 96% initially and 95% after 4 h with the HPLC method, whereas the European and American SPCs both specify a limit of 90% with the SPE cartridge method, although the methods are different.
The European SPC specifies 2 preparation volumes with different activity limits and shelf lives: 1,110 MBq in 10 mL, with a 4-h expiration, and 925 MBq in 4 mL, with a 1-h expiration. The first volume has an equivalent concentration of 111 MBq/mL, and the second has a concentration of 231 MBq/mL. These data lead to issues concerning the concentration effects of 99mTc-MAG3 and eluate volumes. Work done by Millar and O'Brien (6) showed that a preparation containing 1,110 MBq in 10 mL is stable for at least 6 h, whereas Vinberg (2) prepared 99mTc-MAG3 with up to 3,000 MBq in 4 mL and reported acceptable stability over 8 h. The present work suggests that concentrations of 200 and 500 MBq/mL can be prepared without adverse effects on RCP or stability. Taken together, these results suggest that the 99mTc-MAG3 complex is more stable than indicated in the SPC. The shorter shelf life of the 4-mL preparation in the European SPC may be based on work by Shattuck et al. (7), who found a lower initial RCP for 5-mL preparations than for 10-mL volumes, although there was no difference in stability.
For determination of the RCP of 99mTc-MAG3, SPE cartridges are regarded as an inexpensive, easy, and rapid alternative to the HPLC method; in fact, the European SPC describes both methods, although with different limits for acceptable RCP values, as noted above. Work done by Murray et al. (8) showed that the SPE method described in the European SPC yielded consistently lower RCP values than the HPLC method. Vinberg (2) also found that the RCP values were lower with the SPE method than with the HPLC method, with the major impurity being found in the hydrophilic fraction. Hepplewhite and Hesslewood (9) directly compared the European and American SPE methods for both formulations and found a significant difference in the results, with the results from the American method being 4.5% higher. The elution rate of the SPE cartridge also was important; indeed, this property was crucial if valid results were to be obtained, with slower elution rates yielding higher RCP values. An elution rate of 1.5 mL/min was found to be the maximum limit (2). Thus, 10 mL of eluent would require 6.7 min, which is a significant amount of time in a busy radiopharmacy.
The widely used ITLC method developed by Chen et al. (5) is not without problems of its own. Murray et al. (10) found that the ITLC method yielded consistently higher RCP values than the HPLC method because of its inability to differentiate lipophilic impurities from bound 99mTc-MAG3; however, they regarded this result as being acceptable because the difference was predictable and could be used to assess the RCP with suitably tighter acceptance criteria. Other problems with the ITLC method include inconsistent spot size and the frequency of artifacts. Murray et al. also found that the SPE method potentially could yield misleading results because the solvent designed to remove hydrophilic impurities also could remove a portion of bound 99mTc-MAG3. This problem was found to be highly variable, which means that the effect could not be predicted and considered. In contrast, Millar and Hesslewood (1) found that the results obtained with the SPE and HPLC methods correlated very well, although they recognized the weakness inherent in the SPE method, namely, the inability to differentiate among the compounds in each eluted fraction. The SPE system relies on the partitioning characteristics of hydrophilic impurities, 99mTc-MAG3, and lipophilic impurities to separate these compounds in the SPE cartridge, and there is no practical way of checking this separation except by analyzing each fraction with a discerning method such as HPLC. The simplified method developed in the present work appears to show very good correlation with both of the approved SPE methods and with the ITLC method, although this method has yet to be compared with HPLC.
It is clear that an SPE method with a solvent system designed to separate bound 99mTc-MAG3 and hydrophilic and lipophilic impurities is required. It also needs to be user-friendly and thus prevent errors during elution from overenthusiastic operators. Finally, to comply with the ICH Q2B regulations, the method needs to be validated in terms of the parameters described above. Our method has been simplified in terms of solvents and volumes, making it quick and easy for radiopharmacies to set up and use. The system is summarized in Table 2.
The results obtained (Table 3; Fig. 1) show that the 3 SPE methods are comparable. To evaluate the potential for reducing elution volumes, the European and American SPE methods were performed with 10-mL eluates collected in four 2.5-mL fractions. Although there did appear to be some variation in when 99mTc-MAG3 was eluted from the cartridge, the results showed that virtually all of the 99mTc-MAG3 was eluted within the first 2 fractions (5 mL). This finding allows a reduction in elution volumes in the simplified method without compromising the results and in turn reduces the time required. When impure mixtures were prepared by the addition of pertechnetate to 99mTc-MAG3, a linear relationship was observed between nominal RCP values for 99mTc-MAG3 over the range of 0%–100% and the apparent RCP obtained with the simplified method.
Accuracy and precision were determined by comparison with the standard ITLC method developed by Chen et al. (5). The results showed that the simplified SPE method was comparable to the ITLC method. The SDs were small, suggesting that the methods were precise, and the means were similar, showing accuracy of the results. Thus, within the limits of the small sample size, the requirements of the ICH Q2B regulations were fulfilled. In particular, the high correlation between the simplified SPE method and the ITLC method provides confidence in the results.
CONCLUSION
The simplified SPE method meets the requirements of the ICH Q2B regulations and produces results equivalent to those provided by the approved SPE methods in the SPCs and the standard ITLC method but is more rapid to perform, with a corresponding reduction in solvent usage.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine Technology, Inc.
References
- Received for publication February 1, 2006.
- Accepted for publication May 15, 2006.