Abstract
Although our understanding of microorganisms has advanced significantly and antimicrobial therapy has become increasingly available, infection remains a major cause of patient morbidity and mortality. The role of radionuclide imaging in the evaluation of the patient suspected of harboring an infection varies with the situation. For example, in the postoperative patient, radionuclide imaging is complementary to CT and is used to help differentiate postoperative changes from infection. In the case of the painful joint replacement, in contrast, radionuclide studies are the primary diagnostic imaging modality for differentiating infection from other causes of prosthetic failure. Several tracers are available for imaging infection: 99mTc-diphosphonates, 67Ga-citrate, and 111In- and 99mTc-labeled leukocytes. At the moment, in immunocompetent patients, labeled leukocyte imaging is the radionuclide procedure of choice for detecting most infections. There are, unfortunately, significant limitations to the use of labeled leukocytes. The in vitro labeling process is labor intensive, is not always available, and involves direct handling of blood products. For musculoskeletal infection, the need to frequently perform complementary marrow or bone imaging adds complexity and expense to the procedure and is an inconvenience to patients. Considerable effort has therefore been devoted to the search for alternatives to this procedure, including in vivo methods of labeling leukocytes, 18F-FDG PET, and radiolabeled antibiotics. This article reviews the current status of nuclear medicine infection imaging and the potential of a murine monoclonal antigranulocyte antibody, fanolesomab, that is currently under investigation. Upon completion of this article, the reader will be familiar with the physical characteristics and uptake mechanisms of tracers currently approved for infection imaging, the indications for the uses of these tracers, and the characteristics and potential indications for a murine monoclonal antigranulocyte antibody under investigation.
- bone
- infectious disease
- labeled leukocytes
- monoclonal antibodies
- antigranulocyte antibodies
- gallium
- infection
Nuclear medicine plays an important role in the evaluation of patients suspected of harboring infection. Although 99mTc-methylene diphosphonate (MDP), 67Ga-citrate, and 111In-oxine- and 99mTc-hexamethylpropyleneamine oxime (HMPAO)–labeled autologous leukocytes are extremely useful, labeled leukocyte imaging is the current radionuclide gold standard for imaging most infections in immunocompetent patients. Unfortunately, the technique has important limitations. The in vitro labeling process is labor intensive, is not always available, and involves direct handling of blood products. For musculoskeletal infection, the need to perform complementary marrow or bone imaging adds complexity and expense to the procedure and is an inconvenience to patients. A satisfactory in vivo method of labeling leukocytes would overcome many of these problems. Several in vivo leukocyte-labeling methods have been investigated, including peptides and antigranulocyte antibodies/antibody fragments. In addition to reviewing the tracers presently available for infection imaging, as well as their indications, this article explores the potential of an in vivo leukocyte-labeling agent currently under investigation: 99mTc-fanolesomab (LeuTech; Palatin Technologies).
AGENTS
99mTc-MDP
Uptake of 99mTc-MDP depends on blood flow and the rate of new bone formation (1). When performed for osteomyelitis, the study is usually done in 3 phases. Three-phase bone imaging consists of a dynamic imaging sequence, the flow or perfusion phase, followed immediately by static images of the region of interest, which is the blood-pool or soft-tissue phase. The third, or bone, phase consists of planar static images of the area of interest, acquired 2–4 h later. SPECT is performed as needed. Images should be acquired on a large-field-of-view γ-camera equipped with a low-energy high-resolution parallel-hole collimator, using a 15%–20% window centered on 140 keV. The usual injected dose for adults is 740–925 MBq (20–25 mCi) of 99mTc-MDP. The normal distribution of this tracer, by 2 h after injection, includes the skeleton, genitourinary tract, and soft tissues (2).
67Ga
67Ga-citrate has been used for localizing infection for more than 3 decades. 67Ga, which is cyclotron produced, emits 4 principal γ-rays suitable for imaging: 93, 184, 296, and 388 keV. Several factors govern uptake of this tracer in inflammation and infection. About 90% of circulating 67Ga is in the plasma, and nearly all of it is bound to transferrin. Increased blood flow and increased vascular membrane permeability result in increased delivery and accumulation of transferrin-bound 67Ga at inflammatory foci. 67Ga also binds to lactoferrin, which is present in high concentrations in inflammatory foci. Some 67Ga may be transported bound to leukocytes. Direct uptake by certain bacteria has been observed in vitro, and this too may account for 67Ga uptake in infection. Siderophores, low-molecular-weight chelates produced by bacteria, have a high affinity for 67Ga. The siderophore–67Ga complex is presumably transported into the bacterium, where it remains until phagocytosed by macrophages (3).
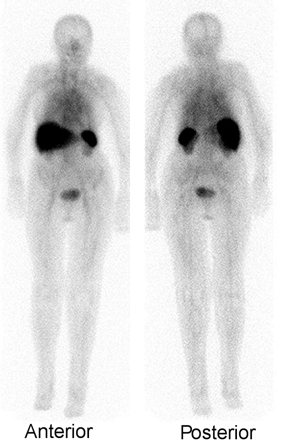
Imaging is usually performed 18–72 h after injection of 185–370 MBq of 67Ga-citrate. A γ-camera capable of imaging multiple energy peaks and equipped with a medium-energy collimator is used. The normal biodistribution of 67Ga, which can be variable, includes bone, bone marrow, liver, genitourinary and gastrointestinal tracts, and soft tissues (Fig. 1) (3).
The normal distribution of activity on 67Ga studies is variable. (A) The skeleton and liver are well visualized and there is faint activity in the colon. (B) Skeletal and hepatic uptake is much less intense, whereas intense activity is present in the proximal colon. (C) The skeleton is well defined, hepatic activity is intense, and the colonic activity, confined to the proximal ascending colon, is faint. (D) Nasopharyngeal activity is prominent, and pancolonic activity is intense.
Labeled Leukocytes
The development of methods to radiolabel inflammatory cells that migrate to sites of infection was a significant milestone in the evolution of radionuclide techniques for imaging infection. Although a variety of in vitro leukocyte-labeling techniques have been used, the most commonly used procedures (and the only approved methods in the United States) make use of the lipophilic compounds 111In-oxyquinoline and 99mTc-HMPAO. The radiolabeling procedure takes about 2–3 h. Approximately 40 mL of whole blood is withdrawn from the patient into a syringe that contains anticoagulant. Because all the cellular components of the blood can be labeled, it is necessary to separate the leukocytes from the erythrocytes and platelets. After withdrawal, therefore, the syringe containing the blood is kept in the upright position for about 1–2 h to promote erythrocyte sedimentation, a process that is facilitated by the addition of hydroxyethyl starch. This process can be accelerated by using hypotonic lysis of the red cells instead of gravity sedimentation (4). After the erythrocytes have been separated, the leukocytes must be separated from platelets. The leukocyte-rich plasma is centrifuged, and the leukocyte “pellet” that forms at the bottom of the tube is removed, incubated with the radiolabel, washed, and reinjected into the patient. The usual dose of 111In-labeled leukocytes is 10–18.5 MBq (300–500 μCi); the usual dose of 99mTc-HMPAO–labeled leukocytes is 185–370 MBq (5–10 mCi) (5).
Uptake of labeled leukocytes is dependent on intact chemotaxis (movement of the cells in response to chemical stimuli), the number and types of cells labeled, and the cellular component of a particular inflammatory response. The labeling of leukocytes, now a routine procedure, does not affect their chemotactic response. A total white count of at least 2,000/mm3 is needed to obtain satisfactory images. Usually, the majority of leukocytes labeled are neutrophils, and hence the procedure is most useful for identifying neutrophil-mediated inflammatory processes, such as bacterial infections. The procedure is less useful for those illnesses in which the predominant cellular response is other than neutrophilic, such as tuberculosis (5).
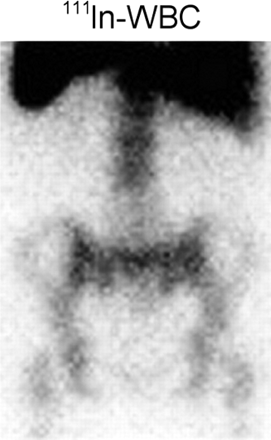
Regardless of whether the leukocytes are labeled with 111In or 99mTc, images obtained shortly after injection are characterized by intense pulmonary activity (Fig. 2). This activity, which clears rapidly, is probably due to leukocyte activation during labeling, which impedes their movement through the pulmonary vascular bed, prolonging their passage through the lungs (6). At 24 h after injection, the usual imaging time for 111In-labeled leukocytes, the normal distribution of activity is limited to the liver, spleen, and bone marrow (Fig. 3).
Immediately after injection of labeled leukocytes, intense activity is normally seen in the lungs. This activity decreases rapidly as the leukocytes leave the lungs, until by 4 h, little activity above background remains. Because of this phenomenon, the lungs should be evaluated only on images obtained more than 4 h, and preferably at 24 h, after injection of labeled cells.
The normal distribution of activity on 111In-labeled leukocyte images acquired 24 h after injection is limited to the liver, spleen, and bone marrow.
The normal biodistribution of 99mTc-HMPAO–labeled leukocytes is more variable. In addition to the reticuloendothelial system, activity is also normally present in the genitourinary tract, large bowel (within 4 h after injection), blood pool, and occasionally the gallbladder (Fig. 4) (7). The interval between injection of 99mTc-HMPAO–labeled leukocytes and imaging varies with the indication; in general, imaging is usually performed within a few hours after injection.
Four- and 24-h whole-body 99mTc-HMPAO–labeled leukocyte images. At 4 h, there is persistent blood-pool activity. Activity is also seen in the liver, spleen, and genitourinary tract. At 24 h, the blood-pool activity has cleared. Activity is present in the bladder and colon, in addition to the liver, spleen, and bone marrow. Compare with Figure 3.
For 111In-labeled leukocyte studies, images should be acquired on a large-field-of-view γ-camera equipped with a medium-energy parallel-hole collimator. Energy discrimination is accomplished by using a 15% window centered on the 174-keV photopeak and a 20% window centered on the 247-keV photopeak of 111In. For 99mTc-labeled autologous leukocyte studies, a high-resolution, low-energy parallel-hole collimator is used with a 15%–20% window centered on the 140-keV photopeak of 99mTc (5).
There are advantages and disadvantages to both 111In- and 99mTc-labeled leukocytes. Advantages of 99mTc-labeled cells include a photon energy that is optimal for imaging using current instrumentation, a high photon flux because more radioactivity is injected, and the ability to detect abnormalities within a few hours after injection. Disadvantages include genitourinary tract activity, which appears shortly after injection, and colonic activity, which appears by 4 h after injection. The instability of the label and the short half-life of 99mTc are disadvantages when delayed 24-h imaging is needed. This occurs in those infections that tend to be indolent and for which several hours may be necessary for accumulation of a sufficient quantity of labeled leukocytes to be successfully imaged (5).
Advantages of the 111In label are a very stable label and a virtually constant normal distribution of activity limited to the liver, spleen, and bone marrow. The 67-h physical half-life of 111In allows for delayed imaging, which is particularly valuable for musculoskeletal infection. There is another advantage to the use of 111In-labeled leukocytes in musculoskeletal infection. Many of these patients require bone or marrow scintigraphy, which can be performed while the patient’s cells are being labeled, as simultaneous dual-isotope acquisitions, or immediately after completion of the 111In-labeled leukocyte study. If 99mTc-labeled leukocytes are used, an interval of least 48 h, and preferably 72 h, is required between the leukocyte and bone or marrow scans.
Disadvantages of the 111In label include a low photon flux, less than ideal photon energies, and the fact that a 24-h interval between injection and imaging is generally required. 99mTc-labeled leukocytes are best suited to imaging acute inflammatory conditions, such as inflammatory bowel disease, whereas 111In-labeled leukocytes are preferred for more indolent conditions such as prosthetic joint infection (5).
99mTc-Fanolesomab
Considerable effort has been devoted to developing in vivo methods of labeling leukocytes, including peptides and antigranulocyte antibodies/antibody fragments. One method makes use of a murine monoclonal IgG1 (Granuloscint; CISBio International) that binds to nonspecific cross-reactive antigen-95 present on neutrophils. Studies generally become positive by 6 h after injection; delayed imaging at 24 h may increase lesion detection (8).
Another agent that has been investigated is a murine monoclonal antibody fragment of the IgG1 class that binds to normal cross-reactive antigen-90 present on leukocytes (LeukoScan; Immunomedics). Sensitivity and specificity of this agent range from 76% to 100% and from 67% to 100%, respectively (8). Neither of these agents is available in the United States.
Another antigranulocyte antibody, an investigational agent currently being evaluated in the United States, is fanolesomab, a monoclonal murine M class immunoglobulin that binds to cluster designation 15 (CD15) receptors present on leukocytes. Several reports have shown specific binding of the monoclonal antibody to human neutrophils (9). This agent presumably binds both to circulating neutrophils that eventually migrate to the focus of infection and to neutrophils, or neutrophil debris containing CD15 receptors, already sequestered in the area of infection (10).
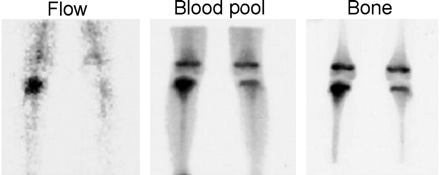
Fanolesomab can be labeled quickly and easily with 99mTc-pertechnetate. About 370–740 MBq (10–20 mCi) of the radiolabeled compound, containing 75–125 μg of antibody, is injected. Activity is initially distributed in the vasculature and eliminated from the blood with a mean linear half-life of 8 h (Fig. 5). Splenic and hepatic activity peak 25–35 min after injection. In contrast to in vitro labeled leukocytes, there is no increased retention of activity in the lungs. The dose-limiting organ is the spleen, which receives an estimated 0.064 mGy/MBq (0.24 rad/mCi), an amount that is considerably lower than the estimated 5 mGy/MBq (18 rad/mCi) for 111In-labeled leukocytes (11).
Whole-body images performed about 3 h after injection of 740 MBq (20 mCi) of 99mTc-fanolesomab. Activity is present in the liver, spleen, bone marrow, and blood pool. Compare this image with the 4-h 99mTc-HMPAO–labeled leukocyte images in Figure 4.
Within 20 min after injection of fanolesomab, a transient drop in the number of circulating leukocytes occurs. No clinical complaints have been associated with this phenomenon, and recovery usually occurs within 45 min (12). Based on available data, the agent is safe, with little toxicity. No serious adverse events occurred among any of more than 400 patients enrolled in multicenter trials. No significant human antimurine antibody (HAMA) titer elevations were observed among 30 healthy volunteers injected with 125 μg of fanolesomab to assess HAMA response. There were no toxic reactions of any kind or changes in vital signs (13).
IMAGING INDICATIONS
Opportunistic Infection
Nuclear medicine plays an important role in the detection of infections unique to the immunocompromised patient, and for most of them, 67Ga imaging is the radionuclide procedure of choice (3,14). Many opportunistic infections affect the lungs, and normal 67Ga findings for the chest exclude infection with a high degree of certainty, especially in the setting of negative findings on chest radiography. In the HIV-positive patient, lymph node uptake of 67Ga is most often due to mycobacterial disease or lymphoma. Focal, or localized, pulmonary parenchymal uptake of 67Ga is usually associated with bacterial pneumonia. Diffuse pulmonary 67Ga uptake is indicative of Pneumocystis carinii pneumonia, especially when the uptake is intense. In addition to its value as a diagnostic test, 67Ga can be used for monitoring response to therapy (3).
Labeled leukocyte scintigraphy, in contrast to 67Ga imaging, is not sensitive for detecting opportunistic infections presumably because most opportunistic infections do not incite a neutrophilic response (Fig. 6) (14). Although no data are presently available, in all likelihood fanolesomab will have a limited role in the evaluation of these patients.
Seen on the 67Ga image is intense, diffuse pulmonary activity, which is typical of Pneumocystis carinii pneumonia. The labeled leukocyte study, in contrast, shows normal findings. 67Ga imaging is superior to labeled leukocyte imaging for detecting most opportunistic infections. (Reprinted with permission of (6).)
Fever of Undetermined Origin (FUO)
FUO is an illness of at least 3-wk duration, with several episodes of fever exceeding 38.3°C and no diagnosis after an appropriate inpatient or outpatient evaluation. FUO has numerous causes, and infection accounts for only about 20%–30% of them. Neoplasms are responsible for about 15%–25%. Other etiologies include collagen vascular disease, granulomatous diseases, pulmonary emboli, cerebrovascular accidents, and drug fever (5). Identifying the source of an FUO is often difficult, and radionuclide studies can provide important information. Several investigators have shown a high negative predictive value (especially for labeled leukocyte imaging) for radionuclide studies (15,16). A negative study excludes, with a high degree of certainty, focal infection as the source of the FUO. To maximize the value of radionuclide studies in the patient with FUO, it is necessary to determine not only where in the diagnostic workup these studies belong but also which radionuclide procedures should be performed and in what sequence. Despite the fact that anatomic modalities such as CT and ultrasonography are no more successful than radionuclide imaging for identifying the source of an FUO, these studies do possess certain advantages. The thorax and abdomen can be surveyed in a matter of minutes. These studies can be performed almost as soon as they are ordered, in contrast to radionuclide studies, which typically require an interval of 24–48 h between tracer injection and imaging. Interventional procedures are increasingly being performed in the imaging suite; if an abnormality is detected, steps such as biopsy and drainage can be taken immediately. In contrast, even abnormal radionuclide studies have to be followed by anatomic imaging for precise localization of an abnormality, before intervention. For these reasons, anatomic modalities are usually the first studies performed in patients with FUO.
When anatomic techniques fail to provide a diagnosis, radionuclide imaging should be performed. Because up to 20% of FUOs are caused by tumor, some investigators have suggested that 67Ga imaging is the preferred radionuclide study. Data indicate that labeled leukocyte imaging is more sensitive early in the course of an illness, whereas 67Ga is more sensitive later in the illness, and the selection of the procedure might also be based on the duration of the illness (17). Although there is no “correct” approach to the radionuclide imaging of the patient with an FUO, we usually begin with an 111In-labeled leukocyte study and follow with 67Ga scintigraphy if needed. The reasons for this are as follows. The energies of the photons emitted by, and the physical half-lives of, the 2 tracers are quite similar. The amount of activity injected for a 67Ga study is typically 10 or more times the amount of activity injected for a labeled leukocyte study. Should the labeled leukocyte study fail to provide a diagnosis, the patient can be injected with 67Ga and scanned 48–72 h later. In contrast, if a labeled leukocyte study is to be performed after the 67Ga study, it is necessary to wait a minimum of 1 wk to obtain diagnostically useful images.
Presently, no data have been published on the role of fanolesomab in the patient with an FUO. Presumably, fanolesomab could replace in vitro labeled leukocyte imaging in this population.
Postoperative Infection
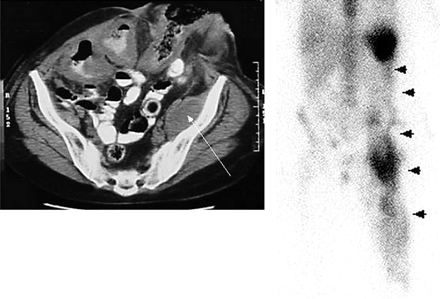
Infection in the postoperative patient is a diagnostic challenge. Anatomic modalities such as ultrasonography and CT cannot always distinguish abscess from other fluid collections, or from tumor, or even from normal postoperative changes (Fig. 7) (18). Although 67Ga can detect intraabdominal infection, the normal presence of large-bowel activity can obscure foci of infection; the frequent need to wait 48 h or more between injection and imaging is another disadvantage. For these reasons 111In-labeled leukocyte scintigraphy, when available, is the preferred radionuclide study. Again, although no data are yet available, the in vitro labeled leukocyte study could potentially be replaced by fanolesomab.
A patient with a history of multiple abdominal surgeries was noted to have a mass on a CT scan of the abdomen and pelvis (arrow). The differential diagnosis included postoperative changes and tumor, but not infection. Abnormal accumulation of labeled leukocytes extends through the left abdomen into the thigh (arrowheads). Multiple abscesses were subsequently drained.
Cardiovascular and Central Nervous System Infections
Echocardiography is a readily available and accurate method for diagnosing bacterial endocarditis, and radionuclide methods play a limited role in the diagnostic workup of this entity (19). Echocardiography is less sensitive, however, for detecting one of the complications of bacterial endocarditis, the myocardial abscess (20). Both 67Ga and labeled leukocyte imaging have successfully detected myocardial abscesses in patients with infective endocarditis (21,22).
A serious complication of bacterial endocarditis is the mycotic aneurysm, which is a dilation of an arterial wall secondary to septic embolization in patients with bacterial endocarditis. The patient with suspected mycotic aneurysm is probably best managed by combining anatomic imaging to localize the aneurysm and labeled leukocyte imaging to determine whether infection is present (23).
The rate of infection after placement of a prosthetic vascular graft is less than 5%; the rate of morbidity and mortality range from about 20% to 75%. Perigraft gas on CT, which is diagnostic of vascular graft infection, is present in only about half of all graft infections (23). Labeled leukocyte imaging is the radionuclide procedure of choice for diagnosing this entity, with a sensitivity of more than 90%; neither duration of symptoms nor pretreatment with antibiotics adversely affects the study (Fig. 8). The specificity of labeled leukocyte imaging is more variable, however, ranging from 53% to 100% (5,24,25). Causes of false-positive results include perigraft hematomas, bleeding, graft thrombosis, pseudoaneurysms, and graft endothelialization, which occurs within the first 1–2 wk after placement (18).
The 111In-labeled leukocyte study demonstrates a linear area of increased activity (arrowheads) in an infected prosthetic vascular graft in the right thigh.
The differential diagnosis of a contrast-enhancing brain lesion identified on CT or MRI includes abscess, tumor, cerebrovascular accident, and even multiple sclerosis. Labeled leukocyte scintigraphy provides valuable information about contrast-enhancing brain lesions. Positive findings indicate that the origin of the brain lesion is almost assuredly infectious; a negative result rules out infection with a high degree of certainty (Fig. 9) (26,27). The technique has some limitations, however. Faint uptake in brain tumors has been observed, and false-negative results in patients receiving high-dose steroids have been reported (26). It is presumed that fanolesomab could replace in vitro labeled leukocyte imaging for both cardiovascular and central nervous system infections.
(A) CT with contrast medium (left) reveals an enhancing ring lesion surrounding a central zone of hypodensity in the gray matter of the parietooccipital region of the left cerebral hemisphere. A transverse SPECT image (right) from the 111In-labeled leukocyte study demonstrates intense focal accumulation of labeled leukocytes in this lesion. An abscess was surgically drained. (B) CT with contrast medium (left) demonstrates an enhancing ring lesion surrounding a central zone of hypodensity in the left parietal lobe of the brain. A transverse SPECT image (right) from the 111In-labeled leukocyte study reveals normal activity within the marrow of the skull but no labeled leukocyte accumulation within the lesion. An astrocytoma was found at operation. (Reprinted with permission of (27).)
Osteomyelitis
Nuclear medicine studies used in the workup of osteomyelitis include 3-phase bone, 67Ga, and labeled leukocyte scintigraphy.
Three-phase bone scintigraphy is the radionuclide procedure of choice for diagnosing osteomyelitis in bones not affected by underlying conditions. Focal hyperperfusion, focal hyperemia, and focally increased bony uptake on delayed (2–4 h after injection) images is the classic presentation of osteomyelitis (Fig. 10) (28). Bone scan abnormalities reflect the rate of new bone formation in general, and consequently, fractures, orthopedic hardware, or the neuropathic joint can yield a positive 3-phase bone scan. In these situations, the bone scan, because of decreased specificity, is less useful. Many of the patients who are referred for nuclear medicine evaluation of osteomyelitis present with underlying bone conditions, and much effort has been devoted to improving the specificity of the radionuclide diagnosis of complicated osteomyelitis. The addition of next-day imaging, referred to as 4-phase bone scintigraphy, improves the specificity of radionuclide bone imaging. Unlike normal bone, in which tracer uptake ceases within about 4 h after injection, uptake in woven, or immature, bone, which is present in osteomyelitis, continues for several hours longer. The result is a higher lesion-to-background ratio on the fourth-phase images than on the third-phase images. The accuracy of 4-phase bone scintigraphy, which is more specific but less sensitive than 3-phase bone imaging, is about 85% (28).
Focal hyperperfusion, focal hyperemia, and focally increased bony activity in the proximal right tibial metaphysis are the classic findings of osteomyelitis.
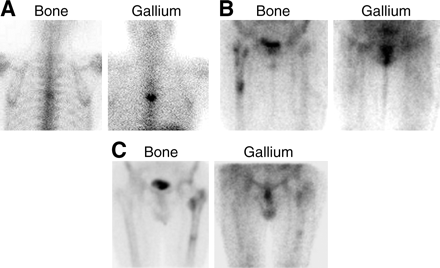
The specificity of bone scintigraphy can also be improved by the addition of 67Ga scintigraphy (28). Because the uptake mechanisms of 67Ga and bone-seeking tracers are different, each study provides information about different aspects of a particular disease process. Combined bone/67Ga imaging is:
Positive for osteomyelitis when distribution of the 2 tracers is spatially incongruent or when the distribution is spatially congruent but the relative intensity of uptake of 67Ga is greater than that of the bone agent (Fig. 11A).
Negative for osteomyelitis when the 67Ga images are normal, regardless of the bone scan findings, or when the distribution of the 2 tracers is spatially congruent but the relative intensity of uptake of 67Ga is less than that of the bone agent (Fig. 11B).
Equivocal for osteomyelitis when the distribution of the 2 radiotracers is congruent, both spatially and in intensity (Fig. 11C).
(A) The spatial distribution of the 2 tracers is similar, but the intensity of uptake is greater on the 67Ga image than on the bone image, and hence the combined study is positive for osteomyelitis. (B) Although periprosthetic activity is increased around a right hip prosthesis on the bone image, the 67Ga findings are normal, and the combined study is negative for infection. (C) The intensity and spatial distribution of both tracers around a left hip prosthesis are similar; thus, the combined study is equivocal for infection.
The overall accuracy of bone/67Ga imaging is about 65%–80% (28). The less than ideal imaging characteristics of 67Ga and the need for 2 isotopes with multiple imaging sessions over several days are disadvantages to the technique.
Labeled leukocytes, which do not usually accumulate at sites of increased bone mineral turnover in the absence of infection, would seem well suited for diagnosing complicated osteomyelitis. The results reported have been variable, however. The primary difficulty in the interpretation of the study is an inability to distinguish labeled leukocyte uptake in infection from uptake in bone marrow. This problem can be overcome by performing complementary bone marrow imaging with 99mTc-sulfur colloid. Both labeled leukocytes and sulfur colloid accumulate in the bone marrow; leukocytes accumulate in infection, sulfur colloid does not. Leukocyte/marrow imaging is positive for infection when there is uptake on the labeled leukocyte image without corresponding uptake on the sulfur colloid image. Any other pattern is negative for infection. The overall accuracy of combined leukocyte/marrow imaging is about 90% (Fig. 12) (28). Combined leukocyte/marrow imaging can be performed in various ways; the precise methodology is dependent on, among other factors, available equipment and may vary from institution to institution. Thus, the protocols that follow are offered as general suggestions, albeit ones that have, in our experience, yielded satisfactory results over the years. Patients should be injected with 370 MBq (10 mCi) of freshly prepared 99mTc-sulfur colloid. We have found that using preparations more than 2 h old frequently results in persistent blood-pool and urinary bladder activity, both of which degrade image quality. The interval between injection and imaging should be at least 30 min. Ten-minute images of the region of interest are acquired on a large-field-of-view γ-camera using a 128 × 128 matrix. If marrow imaging is performed before injection of the 111In-labeled leukocytes, a low-energy, high-resolution parallel-hole collimator and a 15%–20% window centered on 140 keV should be used. If imaging is performed after injection of labeled cells, a 10% window centered on 140 keV should be used, although the rest of the acquisition parameters can remain unchanged. If simultaneous dual-isotope imaging is to be performed, a medium-energy parallel-hole collimator is used, with a 10% window centered on 140 keV, a 5% window centered on 174 keV, and a 10% window centered on 247 keV. Images should again be acquired for 10 min per view using a 128 × 128 matrix.
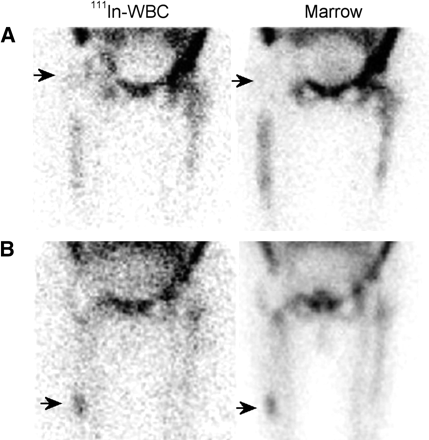
(A) The activity in the proximal left tibia (arrowhead) on the labeled leukocyte image of a patient with Gaucher’s disease could easily be interpreted as consistent with osteomyelitis. The distribution of activity on the bone marrow image is virtually identical however, and the combined study is negative for osteomyelitis. (B) On the labeled leukocyte image, activity is increased in both the proximal and the distal left tibia. The marrow image shows no corresponding activity, and the combined study is positive for multifocal osteomyelitis of the left tibia.
It is important to recognize that no single tracer is equally efficacious in all regions of the skeleton, and the selection of the appropriate study is governed by the clinical question posed.
Spinal osteomyelitis is usually confined to the vertebral body and intervertebral disk; the posterior elements may be involved in up to 20% of cases. MRI, with an accuracy of 90%, is the diagnostic imaging procedure of choice for this entity, and nuclear medicine studies are reserved for those situations in which MRI cannot be performed or is not diagnostic. The current radionuclide procedure of choice for diagnosing spinal osteomyelitis is bone/67Ga scintigraphy; data from our own institution suggest that 67Ga SPECT imaging alone is sufficient (28,29). In contrast to other sites in the skeleton, labeled leukocyte imaging is not useful for detecting spinal osteomyelitis. Although increased uptake is virtually diagnostic, 50% or more of all cases of vertebral osteomyelitis present as areas of decreased or absent activity on leukocyte images (Fig. 13). This photopenia is not specific for vertebral osteomyelitis and is associated with a variety of other conditions such as tumor, infarction, and Paget’s disease (30).
Labeled leukocyte activity is markedly decreased in osteomyelitis of the lower lumbar spine. This pattern, though consistent with, is not specific for, spinal osteomyelitis.
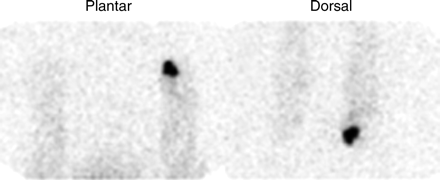
Diabetes mellitus affects about 6% of the U.S. population (31). The most common complication in the diabetic forefoot is the mal perforans ulcer, accounting for more than 90% of all cases of diabetic pedal osteomyelitis. Most patients with pedal osteomyelitis present without systemic illness and lack obvious clinical signs and symptoms, other than ulcer; thus, the diagnosis is overlooked. Imaging studies are routinely used to confirm the diagnosis, and the radionuclide gold standard for diagnosing this entity is labeled leukocyte imaging, with an overall accuracy of about 80% (Fig. 14) (32).
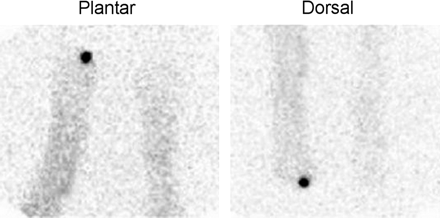
Focally intense activity is present on both the dorsal and the plantar labeled leukocyte images of a diabetic patient with pedal osteomyelitis of the left great toe.
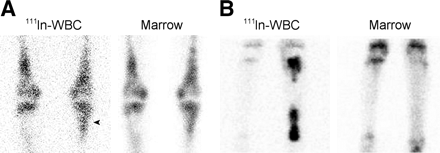
In the mid and hind foot, the most commonly encountered complication of diabetes is the neuropathic joint, or Charcot’s joint. Although the neuropathic joint does not usually become infected, determining if superimposed infection is present, or differentiating the rapidly progressive neuropathic joint from osteomyelitis, is difficult. The striking bony changes that accompany this entity greatly limit the utility of plain radiographs and 3-phase bone scans. It is important to recognize that labeled leukocytes accumulate in the uninfected neuropathic joint and that the presence of such activity cannot automatically be equated with infection. By performing complementary marrow scintigraphy, it is possible to accurately determine whether infection is present (Fig. 15) (33).
Labeled leukocyte and marrow images from a patient with osteomyelitis in Charcot’s joint of the left foot. Spatially congruent activity is present in the distal left tibia on both the labeled leukocyte and the marrow images (arrows), confirming that uptake of labeled leukocytes in this region is due to marrow, not to infection. In contrast, no activity is seen in the left midfoot on the marrow image that corresponds to the activity in this region on the labeled leukocyte study (arrowheads), and hence the uptake of labeled leukocytes in this region is due to osteomyelitis.
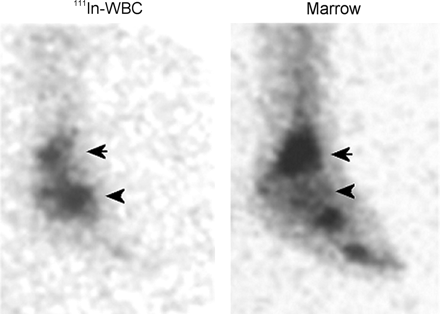
Differentiating the aseptically loosened from the infected joint prosthesis is not always easy, because both entities are remarkably similar, clinically and histopathologically. Aseptic loosening is often caused by an immune reaction to the prosthesis. Histiocytes, giant cells, lymphocytes, and plasma cells accompany the inflammation. Proinflammatory cytokines and proteolytic enzymes are secreted and lead to osteolysis and loosening. These same events may also occur in infection, with one important difference: Neutrophils, usually absent in aseptic loosening, are invariably present in infection. Clinical signs and symptoms, laboratory tests, and joint aspiration are insensitive, nonspecific, or both (34). Radionuclide imaging is perhaps the most useful imaging modality for evaluating the painful prosthesis, and combined leukocyte/marrow imaging, with an accuracy of more than 90%, is the radionuclide procedure of choice for determining whether infection is present (Fig. 16) (35,36).
(A) Subtly increased labeled leukocyte activity is seen in the right hip region (arrow) of a patient with a painful right hip prosthesis. No increased activity is present in this region (arrow) on the marrow image, and the combined study is positive for infection of the prosthesis. (B) Focally increased activity at the tip of the femoral component (arrow) of an aseptically loosened right hip replacement is present on both the labeled leukocyte and the marrow images, and the combined study is negative for infection.
99mTc-Fanolesomab and Osteomyelitis
Initial results suggest that this agent can accurately diagnose osteomyelitis in the appendicular skeleton. In one study, 24 patients with suspected osteomyelitis were imaged up to 2 h after tracer injection. Patients also underwent 111In-leukocyte and 3-phase bone imaging. There were 11 cases of osteomyelitis. Bone scintigraphy was sensitive (100%) but not specific (38%). The 2-h antibody images were sensitive (91%), moderately specific (69%), and comparable to 111In-labeled leukocytes (91% sensitivity, 62% specificity) (Fig. 17). Interpreted together with bone images, the sensitivity and specificity of both the antibody and the 111In-labeled leukocytes improved to 100% and 85%, and 100% and 77%, respectively. In this series, the performance of the antigranulocyte antibody was comparable to that of 111In-labeled leukocytes and, when combined with bone imaging, was more accurate for diagnosing osteomyelitis than were any of the other studies (37).
Both the 2-h fanolesomab and the 24-h labeled leukocyte images reveal increased activity around an infected right knee prosthesis. The distribution of activity on these 2 images is not identical. The fanolesomab image was performed 2 h after injection, whereas the labeled leukocyte image was performed about 24 h after injection. Thus, the fanolesomab image probably reflects an earlier stage of labeled leukocyte accumulation, whereas the leukocyte image reflects a later stage of labeled leukocyte accumulation.
The role of fanolesomab in the diagnosis of osteomyelitis in diabetic patients with pedal ulcers has also been studied (38). Twenty-five diabetic patients with pedal ulcers, 22 in the forefoot and 3 in the midfoot, underwent antibody, 111In-labeled leukocyte, and 3-phase bone imaging. The 1-h fanolesomab, 24-h labeled leukocyte, and 3-phase bone images were interpreted separately and classified as positive or negative for osteomyelitis. The antibody and labeled leukocyte images were also interpreted together with the bone images. The sensitivity, specificity, and accuracy of fanolesomab were 90%, 67%, and 76%, respectively, comparable to those obtained with labeled leukocyte imaging: 80%, 67%, and 72%, respectively (Fig. 18). The antibody was as sensitive as, and significantly more specific (P = 0.004) than, 3-phase bone imaging. Interpreting the antibody together with the bone scan did not change the results, which suggests that the antibody alone is sufficient for diagnosing diabetic pedal osteomyelitis.
One-hour fanolesomab images from a diabetic patient with pedal osteomyelitis of the right great toe.
As encouraging as these initial reports are, some questions must still be answered. Only a small number of prosthetic joints have been studied to date, and the need for marrow imaging is yet uncertain. In most diabetic patients studied, the area of concern was the forefoot. Patients with open, granulating, surgical incisions were excluded from this investigation. Finally, only patients receiving antibiotic therapy for fewer than 7 d were eligible for entry into the study. Consequently, the utility of fanolesomab in Charcot’s joint, in patients with healing surgical incisions, and in patients receiving antibiotics for more than 1 wk is unknown.
Although no data are available, it is reasonable to presume that fanolesomab, because it is essentially another method of performing labeled leukocyte imaging, will probably not be useful for diagnosing spinal osteomyelitis.
Appendicitis
Acute appendicitis presents as vague epigastric or periumbilical pain that increases in intensity and localizes in the right lower quadrant over the course of a few hours. This pain may be accompanied by loss of appetite, nausea, vomiting, and low-grade fever. There is localized tenderness in the right lower quadrant on deep palpation, coughing, or removal of the palpating hand, so-called rebound tenderness. The erythrocyte sedimentation rate and C-reactive protein levels are increased, and leukocytosis may be present. This typical presentation of acute appendicitis is found in only about 50%–60% of patients. In the remainder of the patients, those with atypical appendicitis, the diagnosis is more difficult and may take longer to recognize. These patients are at risk for appendiceal perforation, a serious complication of appendicitis that can result in abscess formation, peritonitis, sepsis, and even death. Complications of delayed or missed diagnosis occur in up to 40% of pediatric patients and in more than 60% of patients older than 50 y (39).
Further complicating matters is the fact that there are entities that mimic appendicitis. Thus the need to avoid underdiagnosis of appendicitis is tempered by the need to avoid overdiagnosis, as surgery is not without risk of morbidity and mortality. The conventional approach to the patient with an atypical or equivocal presentation for acute appendicitis includes either a prolonged in-hospital stay for observation and frequent examination, or imaging studies, or discharge from the hospital with advice to return if symptoms worsen. Ultrasonography has an accuracy of only about 30%. CT, with an accuracy of 93%–94% with use of oral and intravenous contrast media, has limitations including a lengthy time for luminal contrast opacification to reach the area of the appendix, decreased sensitivity in patients with little body fat, and risk of allergic reaction to intravenous contrast media (39). In vitro labeled leukocyte imaging using 99mTc-HMPAO–labeled leukocytes accurately diagnoses early appendicitis. In a study of 124 patients, 58% of positive findings were evident within 1 h and 73% by 2 h after injection of labeled leukocytes. Sensitivity and specificity for acute appendicitis were 98% and 85%, respectively. The in-hospital stay for patients with negative findings decreased from an average of 3.2 d to less than 1 d (40). Similar results have been reported in children (41).
Time is of the essence in the diagnosis of appendicitis, and therefore, in vitro labeled leukocyte imaging has not enjoyed widespread use. Fanolesomab, which is easily prepared and rapidly completed, may be a valuable diagnostic adjunct in atypical appendicitis and may, in fact, serve as a screening test for acute appendicitis (42,43). In an investigation of 49 patients, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were 100%, 83%, 93%, 87%, and 100%, respectively. The average time from injection to visualization of an infected appendix was 9 min (42). In a large multicenter trial, 200 patients with an equivocal presentation of acute appendicitis underwent fanolesomab imaging. Fifty-nine of the patients had histopathologically confirmed acute appendicitis. Sensitivity, specificity, and accuracy were 90%, 87%, and 88%, respectively. The positive and negative predictive values were 74% and 95%, respectively. In 90% of patients with acute appendicitis, study findings were positive within 1 h (Fig. 19). The short time to diagnosis and high negative predictive value make fanolesomab a potentially useful screening tool for suspected acute appendicitis (43).
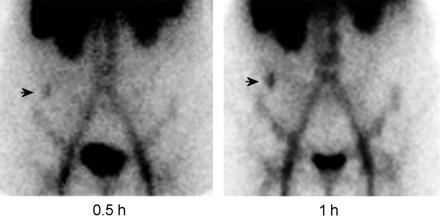
A fanolesomab study, performed on a patient with acute appendicitis, demonstrates focal activity in the right lower quadrant of the abdomen (arrow), which increases in intensity over time.
18F-FDG PET
The high-resolution tomographic images, increasing availability of the agent, and rapid completion of the procedure have spurred considerable interest in 18F-FDG PET for diagnosing infection and inflammation. Although a comprehensive analysis of the role of 18F-FDG PET in infection imaging is beyond the scope of this review, a brief summary of this nascent field is in order. 18F-FDG is carried via glucose transporters into cells. Inflammatory cells have an increased expression of glucose transporters when they are activated. In addition, cytokines and growth factors increase the affinity of glucose transporters for deoxyglucose. The end result is greater 18F-FDG accumulation in infected and inflamed tissues than in normal tissues (44).
18F-FDG PET has long been used in the immunocompromised patient. In the central nervous system, it can help distinguish lymphoma from toxoplasmosis. Outside the central nervous system, although 18F-FDG PET is sensitive for localizing abnormalities, it cannot distinguish benign from malignant diseases (45,46). An area in which 18F-FDG PET will likely have a significant impact is the radionuclide evaluation of the patient with FUO (47). Rather than beginning with labeled leukocyte imaging followed by 67Ga imaging, 18F-FDG PET may, because of its sensitivity, become the initial radionuclide study performed. Labeled leukocyte and 67Ga imaging would follow as needed. The value of 18F-FDG PET in the postoperative patient is not known. Infection cannot reliably be distinguished from tumor, and whether infection can be distinguished from postoperative inflammation is a question that has yet to be answered.
In the evaluation of musculoskeletal infection, data are now emerging that indicate that 18F-FDG PET accurately detects spinal osteomyelitis and could potentially replace 67Ga for this purpose (Fig. 20) (48–50). The role of 18F-FDG PET in the evaluation of the painful joint prosthesis is uncertain. Although initial studies were encouraging, recent data suggest that it may not be possible to distinguish the aseptically loosened prosthesis from the infected one (51,52).
Both 67Ga SPECT and 18F-FDG PET images demonstrate intense activity in a case of osteomyelitis of the lower lumbar spine.
Presently, little is known about the utility of 18F-FDG for the evaluation of diabetic foot infections.
SUMMARY
Nuclear medicine plays an important role in the diagnosis of a multitude of infections. In some situations, such as in the postoperative patient, this role is complementary to that of other imaging studies. In other conditions, such as the prosthetic joint, radionuclide studies are the primary imaging modality for determining whether infection is present. The tracers presently available for localizing infection are limited to bone, 67Ga, and in vitro labeled leukocyte imaging. It is likely, however, that in vivo labeled leukocyte imaging with agents such as fanolesomab, 18F-FDG PET, and perhaps even radiolabeled antibiotics will soon be added to this array.
Footnotes
For correspondence or reprints contact: Christopher J. Palestro, MD, Division of Nuclear Medicine, Long Island Jewish Medical Center, 270-05 76th Ave., New Hyde Park, NY 11040.
E-mail: palestro{at}lij.edu
↵* NOTE: FOR CE CREDIT, YOU CAN ACCESS THIS ACTIVITY THROUGH THE SNM WEB SITE (http://www.snm.org/ce_online) THROUGH JUNE 2005.