Abstract
Objectives:The purpose of this study was to verify the accuracy and reproducibility of a multiwell counter to assess its suitability for use within human PET studies in which metabolizing 11C tracers are used. Such tracers often require metabolite analysis for deriving plasma metabolite-corrected input curves. High-pressure liquid chromatography (HPLC) with on-line activity measurement is often unreliable for later plasma samples due to the poor sensitivity of the on-line activity detector. Fraction collector obtained HPLC samples that are counted in a separate high-sensitivity well counter can be an alternative to overcome poor counting statistics.
Methods:Several experiments to evaluate background counting, reproducibility, and linearity were performed to validate the accuracy, precision, and detection limits of the well counter. In addition, measurements on a series of samples resembling activity profiles as seen within human 11C-flumazenil studies were performed to evaluate the performance of the well counter for clinically relevant data.
Results:The tests proved that the well counter detection limit, linearity, and reproducibility were more than sufficient in circumstances as seen during patient studies for samples with both high and low activity.
Conclusion:The use of a multiwell counter is a good alternative for the on-line activity detector of the HPLC, allowing derivation of plasma metabolite fractions with high accuracy and reproducibility.
Quantification of PET studies using 11C-labeled ligands usually requires measurement of the time course of the labeled ligand in arterial plasma (1). Because the majority of ligands are metabolized within the body, total radioactivity in arterial plasma needs to be corrected for the presence of radioactive metabolites. Separation of parent compound and metabolites is routinely performed using high-pressure liquid chromatography (HPLC) and with radioactive fractions being measured in a multiwell counter. An alternative is on-line detection within the HPLC system.
Due to the short half-life of 11C (~20 min) and (often rapid) clearance from the circulation, actual radioactivity concentrations at the end of a PET study (typically 60 min) can be very low. As quantification of the ligand study might be critically dependent on the shape of the tail of the input function, HPLC fractions need to be counted with high accuracy and precision. Because sensitivity of available on-line HPLC activity detection systems is too low, use of a fraction collector and well counter is required, in particular for accurate determination of metabolite fractions in later plasma samples.
The purpose of the present study was to assess and validate a routine multiwell γ-counter for measuring HPLC fractions of plasma samples that are typical for human 11C-flumazenil studies.
MATERIALS AND METHODS
In this study, an LKB Wallac 1470 Wizard (Perkin Elmer Life Science) was used. This is a multiwell γ-counter equipped with 5 wells. The counter automatically compensates for spillover from one well to the others. Automatic background correction is also standard, whereas correction for decay during the counting process is optional. In the present study, decay correction was applied to the beginning of counting.
The counter was validated for the following parameters: (a) background reproducibility of each separate well individually, (b) reproducibility of each well individually using a high-energy radionuclide point source, (c) reproducibility of both background and point source readings for all wells combined, (d) reproducibility of counting a series of samples resembling an HPLC curve, (e) linearity of the counter within the range of count densities seen in clinical 11C tracer studies.
As the efficiency of the Wizard 1470 well counter decreases with increasing γ-energy, its reproducibility for PET tracers should ideally be assessed for an energy of 511 keV. Because a suitable PET radionuclide calibration source with long half-life was not available, a 7-kBq 137Cs point source (energy, 662 keV; half-life, 30.12 y) was used as a stable, high-energy calibration source for reproducibility tests. All counting measurements were done for 1 min.
To verify reproducibility of HPLC sample measurements, 57Co (γ-emitter; half-life, 270 d; primary energy levels: 85% 122 keV, 11% 136 keV) was the most convenient radionuclide available with a half-life long enough to discard corrections for decay between all measurements. A 57Co-vitamin B12 Schilling test capsule (Draximage) was diluted in a volume of approximately 10 mL. This solution was used to create simulated HPLC profiles comparable with the profiles for both early (high concentration) and late (low concentration) 11C-flumazenil plasma samples (2). Fractions of the 57Co solution were divided over 40 tubes to create the profiles. All fractions were then diluted to a total volume of 1.5 mL to avoid geometry differences and to simulate the number of tubes and volumes obtained during the actual HPLC procedure of human plasma samples (3,4) and placed in the well counter to be counted for 1 min during each counting cycle.
Linearity of the well counter was assessed by measuring a range of radioactivity concentrations using 2 series of 10 tubes each. Predetermined volumes of a 57Co-containing stock solution were diluted to 1.5 mL. One series contained a low range of radioactivity concentrations (9.7 × 10−4 to 5.8 × 10−2 kBq) giving a count range of 40–2,400 counts per minute (cpm). The second series contained higher concentrations (2.1 × 10−2 to 1.4 kBq) resulting in a higher count range of 870–58,250 cpm. All samples were counted once within the same well for 1 h and a second time for 1 min.
RESULTS
Background reproducibility measurements (counting period, 1 min) of the wells using 11C settings yielded an average of 176 ± 14 counts (n = 315, each individual well measured 63 times)—that is, the coefficient of variation (COV) was 8%.
The 137Cs point source was measured 49 times in each well—thus, 245 times in total. The average activity of the source was 28,930 ± 383 counts—that is, with a COV of 1.3%.
Detailed results are provided in Table 1.
Repeated Well Counter Measurements of Background Levels and a 137Cs Source
The 2 simulated HPLC curves using 57Co were counted 55 times each. Examples of high- and low-activity curves are shown in Figures 1 and 2, respectively. The total amount of activity within the 40 tubes simulating the high-activity plot had an average of 61,968 cpm; the SD was 267 cpm (i.e., COV = 0.4%). The low-activity plot contained on average 1,462 cpm, this amount of activity also divided over 40 tubes; the SD was 73 cpm, corresponding to a COV of 5.0%.
Simulated HPLC curve resembling an early 11C-flumazenil plasma sample. The curve was generated using a 57Co stock solution. Shown is the mean cpm for 55 repeated measurements together with the SD corresponding with each measurement. (SD bars are almost invisible as they are the same size or smaller than the size of the circles).
Plot of the mean (n = 55) minimum and maximum cpm values for a simulated HPLC curve resembling a late 11C-flumazenil plasma sample. The curve was generated using a 57Co stock solution. Shown is the mean cpm for 55 repeated measurements together with the SD corresponding with each measurement.
The simulated HPLC curves contained 3 activity peaks comparable with those seen in clinical 11C-flumazenil plasma curves. Peak integration was performed for all 55 measurements of both curves to assess peak integration reproducibility. More detailed results of these experiments are given in Table 2.
Well Counter Reproducibility for Simulated (Flumazenil) HPLC Metabolite Curves (55 Repeated Measurements)
The linearity of the well counter was verified using linear regression of both high- and low-activity standard curves. For the 1-h counting periods, linear regression of the cpm versus the volume of 57Co solution yielded a correlation coefficient of 0.99975 for the low-activity range and 0.99998 for the high- activity range. When the counting time was reduced to 1 min, the coefficients became 0.99868 for the lower cpm range and 0.99986 for the higher cpm range. (Fig. 3)
Measured cpm as a function of the volume of 57Co in 1.5 mL for 2 concentrations of 57Co stock solution. The cpm values for the low-activity curve are on the right y-axis, and those for the high-activity value curve are on the left y-axis. All samples were counted in the same well with a counting time of 1 min for each sample. The correlation coefficients for the lines were 0.99868 and 0.99986 for the low- and high-activity curve, respectively.
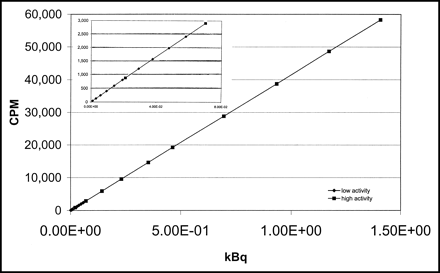
In Figure 4, both standard curves are shown together as cpm versus kBq. In addition, the lower range of cpm values is shown separately as an insert.
Measured cpm as a function of the absolute amount of radioactivity (kBq). The insert shows an enlargement of the low-activity range.
DISCUSSION
Because HPLC profiles of plasma samples may vary from study to study, it is possible that the highest peaks may appear in different wells of the well counter. Therefore, all wells should perform similarly—that is, within a narrow COV—to avoid significant differences between studies. As can be seen from Table 1, the reproducibility for each individual well differed only slightly at background levels. Counting a 137Cs source, the COV for individual wells was <0.7%.
In practice, however, samples will be counted in different wells and, therefore, variability between all wells should be considered. The COV of all wells combined was 1.3%, which is twice as high as the COV for individual wells. Nevertheless, this variation is small compared with the potential errors involved in preprocessing the samples.
In general, linearity of a well counter is limited to a certain activity range. In particular, one should be aware of nonlinearity at high counting rates. In human 11C-flumazenil studies, however, no single sample was obtained that fell outside the linear range of the well counter. However, for every tracer, it should be confirmed that all samples remain within this linear range.
With 57Co-simulated HPLC profiles, representative for 11C-flumazenil studies early after injection, a COV of <2% for individual peaks was obtained with an expected COV for the parent compound peak of <1%. As expected, for later times, when the total amount of activity has decreased (decay + biologic clearance), the predicted COV for individual peaks was found to deteriorate to a range of 5%–9% with an expected COV for the parent compound peak around 9%. For kinetic modeling, only the parent flumazenil in arterial plasma is required as an input function. Based on the simulations described in this study, the uncertainty in the resulting input function can be determined for individual studies. This, in turn, can be used to determine uncertainties in fitted parameters.
CONCLUSION
In this study, a comprehensive set of tests was used to validate a multiwell γ-counter for measuring HPLC samples. Based on simulated concentrations, precision of estimated metabolite peaks was found to be in the range of <1%–10% depending on the peak height and time of measurement. These data are important for determining resulting errors in kinetic parameters when metabolite-corrected plasma data are used as an input function. The counter is suitable for use within kinetic studies for fast-decaying nuclides, though implementation within such protocols requires precautionary actions within the standard operation procedures, due to the rapid decay, to obtain optimal results.
Footnotes
For correspondence or reprints contact: Henri N.J.M. Greuter, PET Center, VU University Medical Center, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands.
E-mail: HNJM.Greuter{at}VUmc.NL