Abstract
Objective:To determine and compare the stability of 99mTc-ECD and stabilized 99mTc-HMPAO when stored in syringes over an 8-h period.
Methods:99mTc-ECD and stabilized 99mTc-HMPAO were prepared according to the manufacturers’ protocols, with the following exception: eluate less than 60 min old was used to prepare 99mTc-HMPAO rather than the recommended 30 min. Once prepared, 185 MBq (5 mCi) of both products were drawn into 5-mL syringes and allowed to sit at room temperature. At 2, 4, 6, and 8 h after preparation, the radiochemical purity (RCP) of the contents of the syringes was determined and compared to the RCP of the products in vials. Retention of activity of each product in syringes was also evaluated by measuring activity remaining in each syringe (and filter, in the case of 99mTc-HMPAO) after expressing its contents.
Results:The RCP of stabilized 99mTc-HMPAO stored in syringes decreased from a mean of 87.7% at 2 h to 74.0% at 8 h after preparation. In contrast, 99mTc-ECD retained an RCP of greater than 94% throughout the time tested. The impurity that appeared to increase over time with 99mTc-HMPAO was found to be sodium pertechnetate. Total retention of activity remaining in the syringe and filter ranged from 11.6% at 2 h to 9.5% at 8 h for 99mTc-HMPAO; the syringe itself retained less than 5% of the total activity at all time periods. 99mTc-ECD exhibited 6.2% to 11.3% retention of activity in the syringe. The sorption of sodium pertechnetate to the syringe for the same time period was less than 1%.
Conclusions:99mTc-ECD is a more stable product than stabilized 99mTc-HMPAO in a syringe. Both products demonstrate retention of radioactivity in the syringe. Some of this retention may denote sorption of the products to plastic.
SPECT has become an important tool in the study of epileptogenic foci in those patients with refractory focal epilepsy (1–3). The radiopharmaceuticals most commonly used in North America for this purpose are technetium (99mTc)-labeled bicisate, or ethylcysteinate dimer (ECD) (Neurolite; DuPont Pharmaceuticals Company, Billerica, MA), and stabilized 99mTc-exametazime, or hexamethylpropyleneamine oxime (HMPAO) (Ceretec; Amersham Healthcare, Arlington Heights, IL). For a patient to be injected during the ictal phase, the radiolabeled product should be prepared in advance and stored in a shielded syringe next to the patient’s bedside.
From a pharmaceutical viewpoint, the stability of an injectable product should be evaluated if the product is transferred to and stored in another container for extended periods of time. In addition, the product should be tested for compatibility with its container before use (4,5). There is always a possibility that lipophilic products such as cerebral perfusion agents could interact with the syringe materials over time. Therefore, the intent of this study was to determine the radiochemical stability and retention of 99mTc- ECD and stabilized 99mTc-HMPAO in syringes over an 8-h period.
Materials and Methods
99mTc-ECD was prepared as follows. To vial B (buffer/reaction vial), 3.7 GBq (100 mCi) sodium pertechnetate in 2 mL sterile saline were added. Only eluate from a generator eluted in the previous 24 h was used. Vial A was reconstituted with 3 mL sterile saline. The contents were gently agitated, and within 30 s, 1 ml of vial A was transferred to vial B. The mixture was allowed to sit at room temperature for 30 min. The contents were examined for particulates. Radiochemical purity (RCP) of the solution was determined, according to the manufacturer’s package insert (6), by thin-layer chromatography using Baker-Flex silica gel IB-F plates (JT Baker Chemical Co., Phillipsburg, NJ) and ethyl acetate.
Stabilized 99mTc-HMPAO was prepared and tested in accordance with the manufacturer’s insert (7), with the exception that pertechnetate was used within 1 h of elution from a generator that was eluted in the previous 24 h. Methylene blue, 0.5 mL, was added to 4.5 mL buffer solution (stabilizing solution). The reaction vial was reconstituted to 5 mL with 1.85 GBq (50 mCi) sodium pertechnetate in saline to which was added 2 mL of the stabilizing solution. The product was then allowed to incubate for 30 min at room temperature. The presence of free pertechnetate and hydrolyzed technetium was determined by using saline and methyl ethyl ketone, and silica gel–impregnated glass fiber (ITLC-SG) paper. The percentage of secondary HMPAO was calculated using acetonitrile-to-water ratio of 50:50 and Whatman #1 paper (Whatman Biosystems, Kent, U.K.), as described in the manufacturer’s insert.
Four latex-free syringes (Becton-Dickinson, Franklin Lakes, NJ) were prepared for each product immediately after quality control was completed, and each 5-mL syringe contained 185 MBq (5 mCi) activity. The syringes were stored at room temperature. RCP of the products was determined at 2, 4, 6, and 8 h after drawing up the doses into the syringes. The purity of the products in the syringes was compared with the RCP of the products in the vials for the same time period.
Retention of the products in the syringes was determined as follows. At the designated times, the full syringes were assayed in a dose calibrator, after which the syringe contents were expressed into a vial. The empty syringes were reassayed in the dose calibrator and the remaining activity compared with that of the full syringes. The needles (and 0.45-μm filter, for stabilized 99mTc-HMPAO) were then removed and the syringes reassayed. The activity in the vial, empty syringe, needle, and filter (where applicable) was totaled and compared with the activity in the syringe before removal of the radiolabeled product, to ensure that geometry was not an influencing factor. For 99mTc-HMPAO, the radioactivity remaining in the filter was assayed separately. Both the RCP and the retention experiments were repeated 10 times for each product.
Controls were prepared by drawing up 185 MBq (5 mCi) of sodium pertechnetate into 5-mL syringes. The contents of the syringes were expressed at the selected times, and each syringe and filter was measured in the dose calibrator to assess the amount of radioactivity remaining.
The Student’s t test was used to compare results between the means of all groups. A value of P < 0.05 was considered statistically significant.
Results
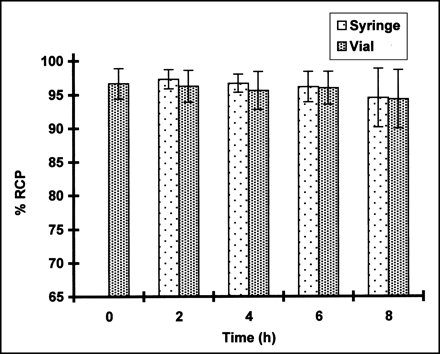
As shown in Figure 1, the RCP of 99mTc-ECD in the syringe and vial remained greater than 94% at all time periods tested. No statistically significant difference in the RCP was observed during the study, regardless of whether the product was stored in a syringe or vial. In contrast, the mean RCP of 99mTc-HMPAO decreased in both the vial and syringe with time (Fig. 2). The initial purity of 99mTc- HMPAO in the vial was observed to be 91.5% at the time of preparation, decreasing to 81.4% at 8 h after preparation. The product deteriorated more rapidly in the syringe, resulting in a mean RCP of 74.0% at the end of the study.
RCP of 99mTc-ECD in syringe and vial over time (mean ± SD; n = 10). No significant difference was observed between RCP of samples at any time period regardless of container used.
RCP of 99mTc-HMPAO in syringe and vial over time (mean ± SD; n = 10). Syringe vs. vial: no significance for 4-h sample; otherwise, significance of at least P < 0.01. Between syringes: no significance between 2- and 4-h samples; P < 0.001 between other samples. Between vials: no significance between 4- and 6-h or between 6- and 8-h samples; otherwise, significance of at least P < 0.05.
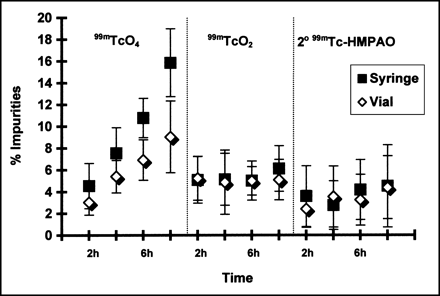
The predominant impurity corresponding with the decrease in RCP of 99mTc-HMPAO was found to be 99mTc- sodium pertechnetate (Fig. 3). 99mTc-sodium pertechnetate accounted for 3% of the activity in the vial, versus 4.5% in the syringe 2 h after preparation. After 4 h this difference became statistically significant, with 5.4% free pertechnetate in the vial compared with 7.6% in the syringe (P < 0.05). At 8 h after preparation, the percentage of pertechnetate in the syringe was almost twice that found in the vial (15.9% in the syringe vs. 9% in the vial; P < 0.001).
% Radiochemical impurities in 99mTc-HMPAO over time (mean ± SD; n = 10). 99mTcO4: no significant difference between 4- and 6-h sample, or between 6- and 8-h sample in vial; otherwise, P < 0.05 at all other time periods in vial, in syringe, and between vial and syringe. 99mTcO2 and 2o99mTc-HMPAO: no significance between samples of any time period.
The radiochemical impurities of reduced, hydrolyzed technetium and secondary HMPAO did not change throughout the 8 h. 99mTcO2 represented 5.1% to 6% of the activity for both containers, whereas the percentage of secondary HMPAO ranged from 2.4% to 4.5%. These results were not statistically significant.
The retention characteristics of the products in syringes are provided in Table 1. The mean percentage of radioactivity remaining in the syringe/filter apparatus containing 99mTc-HMPAO was observed to be 11.6% at 2 h, decreasing to 9.5% at 8 h after preparation. With the filter removed, the syringe was found to contain 4.6% to 4.9% of the initial activity. In contrast, 99mTc-ECD retained 10.1% to 11.3% of initial activity in the syringe at 2, 4, and 6 h after preparation, with a decrease to 6.2% at 8 h. In comparison, sodium pertechnetate exhibited less than 1% retention in the syringe over the 8-h time period, and approximately 4% of the pertechnetate remained in the filter.
Percentage of 99mTc-ECD and 99mTc-HMPAO Retained in Syringe and Filter
Discussion
To effectively determine the foci of seizures in refractory epilepsy, patients are admitted to the hospital, taken off anticonvulsant medication, and monitored for seizure activity. Brain-imaging agents are injected within 20 s of seizure onset. The optimum agent for this type of procedure should be one that can be prepared early in the morning and remain stable in a syringe for at least 8 h. This experiment was performed to determine which agent, 99mTc-ECD or stabilized 99mTc-HMPAO, would be best suited for this type of procedure.
99mTc-HMPAO appears to be more sensitive than 99mTc- ECD to the transfer to and storage in another container. Although 99mTc-ECD maintained its stability of greater than 90% over 8 h in both the vial and syringe, stabilized 99mTc- HMPAO retained an RCP greater than 80% only when stored in a vial. Storage of the product in a syringe resulted in suboptimal RCP 8 h after preparation. The transfer of stabilized 99mTc-HMPAO to a syringe apparently increases the rate of reoxidation of bound technetium to sodium pertechnetate.
It is unlikely that the observed decrease in RCP of stabilized 99mTc-HMPAO 6 to 8 h after preparation would have improved had the manufacturer’s recommendation been followed with respect to the use of generator eluate within 30 min of elution. Ballinger et al. (8) showed that the initial stability of nonstabilized 99mTc-HMPAO was not affected either by generator ingrowth of 24 h or less, or by use of eluate within 4 h of elution. The 60-min elution limit used in this study was employed solely because of workload considerations in the radiopharmacy.
It should be noted that an 8-h study period is longer than the recommended expiration time for the prepared products (6 h for 99mTc-ECD; 4 h for stabilized 99mTc-HMPAO). In addition, the RCP of both products exceeded the manufacturers’ requirements at expiration, regardless of the container used. To strictly comply with the manufacturer’s package insert, it would be necessary to replace stabilized 99mTc-HMPAO every 4 h (and 99mTc-ECD at 6 h), regardless of the in vitro stability. It should also be noted that stability studies using a small sample size of 10 vials might be inadequate to establish an expiration time longer than that specified by the manufacturer.
Retention studies were performed to determine whether either product would be lost in the syringe, thus reducing the dose available for injection into the patient. Plastics that contain little or no phthalate plasticizers (such as polypropylene syringes used in this study) typically do not readily absorb lipid-soluble drugs (9). Adsorption of a product, however, must also be considered given that a clinically relevant amount of product could be easily removed from the solution. The risk of sorption increases with the length of time that the product is exposed to the surface. Any pharmaceutical product that is housed in a container for a prolonged period of time should be tested for compatibility with that container before use.
Both 99mTc-ECD and stabilized 99mTc-HMPAO retained approximately 10% of their total activity in the syringe (or syringe/filter, in the case of 99mTc-HMPAO). With 99mTc-HMPAO the activity was distributed equally between the syringe and the filter. Filters are not required for the injection of 99mTc-ECD and therefore were not used in the 99mTc-ECD retention studies. 99mTc-ECD, however, exhibited significantly higher retention in the syringe compared with that of either 99mTc-HMPAO or the control syringes containing 99mTcO4 (99mTc-ECD; 6.2%–11.3%; 99mTc-HMPAO, 4.6%–4.9%; 99mTcO4, 0.5%–0.9%; P < 0.01). Higher retention of 99mTc-ECD is not likely caused by the lipophilic nature of the chemical, given that ECD is less lipophilic than HMPAO, based on the higher octanol/water partition coefficient of HMPAO (log P = 1.9, HMPAO; log P = 1.64, ECD) (10). The amount of 99mTc-ECD or 99mTc-HMPAO remaining in the syringe was observed to be significantly higher than that of the 99mTcO4 control. This likely reflects some form of sorption to the syringe.
A statistical difference was also observed between the radioactivity retained in the filters containing 99mTc-22HMPAO at 2, 4, and 6 h after preparation and that of the control filters (P < 0.001). This observed retention might represent adherence of the product to the filter. A portion of this activity may also reflect the lack of flushing of the syringe/filter after expression of the product from the syringe rather than interaction of the product with the filter. Flushing the syringe/filter with saline would have determined whether activity was irreversibly bound to the container, but this would not have represented the clinical situation (syringes are not flushed during injection because a bolus injection is desirable for the ictal study).
Conclusion
From a pharmaceutical point of view, 99mTc-ECD would be the preferred agent for use in ictal studies. The product displays greater stability than stabilized 99mTc-HMPAO when stored in a syringe for at least 8 h. Therefore, 99mTc-ECD is required to be prepared only once during the day. In contrast, two preparations of 99mTc-HMPAO would be required to provide availability of a product of specified radiochemical purity throughout an 8-h period. This would increase the cost of the procedure as a result of the need for additional product, generator eluate, and labor.
Retention studies indicate that 10% of the product remains in the syringe/filter, regardless of the product selected. Although sorption of the brain agents does appear to occur, the radiochemical stability of the product should be the main determining factor in the selection of the most appropriate agent for seizure studies.
Acknowledgments
The authors thank DuPont Pharma for partial sponsorship of this project.
Footnotes
For correspondence or reprints contact: Ingrid Koslowsky, Nuclear Medicine, Foothills Medical Centre, 1403 29th Street NW, Calgary, Alberta T2N 2T9, Canada; Phone: 403-670-1160; Fax: 403-670-1687; E-mail: ingrid.koslowsky{at}CalgaryHealthRegion.ca.