Abstract
The heart’s innervation relies on a delicate balance between the sympathetic and parasympathetic nervous systems, each using distinct neurotransmitters to regulate heart rate, contractility, and vascular tone. The sympathetic division primarily uses norepinephrine, whereas the parasympathetic division operates through acetylcholine. A range of diseases, through intrinsic and extrinsic mechanisms, can disrupt these neural pathways, resulting in autonomic dysfunction. This review highlights intrinsic causes such as dysautonomias, amyloidosis, diabetes mellitus, Parkinsonian syndromes, and Lewy body dementia, as well as extrinsic factors such as heart failure, myocardial ischemia, infarction, and drug-induced cardiotoxicity. This article examines the effects of various conditions on cardiac sympathetic innervation and highlights how 123I-radiolabeled metaiodobenzylguanidine (MIBG), a norepinephrine analog, can target the cardiac sympathetic nervous system for early detection and disease characterization. Currently, variability in cardiac 123I-MIBG imaging protocols across institutions leads to inconsistencies in image acquisition and interpretation, limiting the establishment of universal benchmarks for distinguishing normal from abnormal cardiac sympathetic innervation. To address this, we propose a simple, clinically useful, standardized protocol based on European Association of Nuclear Medicine guidelines and the AdreView Myocardial Imaging for Risk Evaluation in Heart Failure trial, incorporating both qualitative and semiquantitative methods for disease assessment and highlight cutoff values for some pathologies that can assist in visual interpretation. Standardizing these protocols will enhance the consistency, reliability, and diagnostic accuracy of 123I-MIBG imaging, improving clinical decision-making and optimizing patient outcomes.
- cardiac 123I-MIBG
- sympathetic nervous system imaging
- cardiac autonomic dysfunction
- dysautonomia
ANATOMY AND PHYSIOLOGY OF CARDIAC INNERVATION
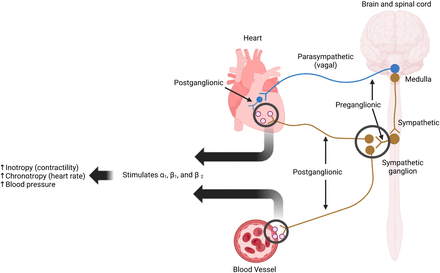
The heart is innervated by the autonomic nervous system, comprising the sympathetic and parasympathetic systems, which regulate chronotropy, lusitropy, dromotropy, and inotropy (1). Sympathetic nerves release catecholamines such as norepinephrine to stimulate adrenergic receptors, increasing heart rate and contractility, whereas parasympathetic nerves release acetylcholine to stimulate muscarinic receptors, decreasing heart rate and contractility (1–5). Sympathetic signals originate in the brain, travel through the spinal cord, and exit at T1–T4, synapsing with postganglionic fibers near the vertebral column that primarily target the ventricles (Fig. 1) (1,3). Parasympathetic fibers originate in the medulla, traverse the vagus nerve, and synapse within the heart, mainly innervating the atria to regulate nodal function (Fig. 1) (1,3).
Sympathetic (brown) and parasympathetic (blue) innervation of heart and systemic vasculature. Postganglionic sympathetic neurons are seen innervating heart and systemic vasculature.
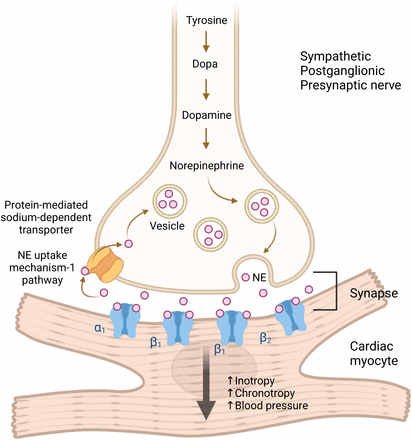
Norepinephrine, derived from tyrosine, is synthesized and stored in presynaptic vesicles at high concentrations (Fig. 2) (3,4,6). On stimulation, it is released into the synaptic space, binding to α-1, β-1 (the primary cardiac adrenergic receptor), and β-2 receptors on cardiac myocytes, increasing heart rate and contractility (4,7). Most norepinephrine is recycled and stored via the norepinephrine uptake-1 pathway or catabolized (3). This pathway helps regulate synaptic norepinephrine levels, preventing excessive catecholamine effects on the heart (Fig. 2).
Diagram of sympathetic neuronal synapse with cardiac myocyte, demonstrating norepinephrine (NE) synthesis, storage, release, and reuptake.
CARDIAC AUTONOMIC DYSFUNCTION
Cardiac sympathetic dysfunction can arise from intrinsic nerve abnormalities or external disease processes, leading to neurohormonal imbalances, myocyte damage, or sympathetic denervation (1,3,4,8,9). Regardless of the cause, this dysfunction results in systemic dysautonomia, manifesting as heart rate irregularities, blood pressure instability, arrhythmias, and impaired diastolic relaxation (1). We will discuss the following intrinsic and extrinsic causes of cardiac sympathetic dysfunction.
Intrinsic Mechanisms
Dysautonomias
Dysautonomia, or autonomic dysfunction, encompasses congenital and acquired disorders that disrupt autonomic functions such as blood pressure, temperature regulation, breathing, and heart rate (9–11). Its diverse symptoms complicate diagnosis and treatment. Dysautonomia exists in primary (inherited or idiopathic) and secondary forms. Primary dysautonomia has been observed in Ashkenazi Jewish and Eastern European populations (10). Secondary dysautonomia arises from systemic conditions such as amyloidosis, Parkinsonian syndromes, malignancies, and inflammatory diseases, including long coronavirus disease 2019 (10,11).
Amyloidosis
Amyloidosis involves misfolded protein aggregates infiltrating organs, including the heart, leading to a poor prognosis with risks of heart failure, arrhythmias, and restrictive cardiomyopathy (12–14). It also causes dysautonomia due to sympathetic denervation and conduction tissue infiltration. Cardiac dysautonomia is most common in hereditary transthyretin-related (ATTR) and immunoglobulin light chain amyloidosis but not in wild-type or senile ATTR (13).
Diabetes Mellitus
Diabetes mellitus is a common cause of intrinsic cardiac autonomic dysfunction, though its exact pathology remains unclear (1,3,6,15–19). It leads to autonomic denervation, primarily affecting distal axons, through mechanisms such as glycosylation and ischemia (1). Although parasympathetic involvement has been demonstrated, its impact on sympathetic signaling is complex (1).
Parkinsonian Syndromes
Parkinsonian syndromes are neurologic disorders characterized by dopaminergic neuron degeneration, including Parkinson disease (PD), multiple system atrophy (MSA), progressive supranuclear palsy, corticobasal syndrome, and dementia with Lewy bodies (DLB) (20). In addition to neuron loss, some syndromes exhibit cardiac dysautonomia and sympathetic denervation (21). MSA affects preganglionic neurons, preserving cardiac sympathetic innervation, whereas PD impacts postganglionic neurons, leading to cardiac sympathetic denervation (21–23).
Extrinsic Mechanisms
Heart Failure
Heart failure, most commonly caused by ischemia, leads to neurohormonal dysregulation, activating the renin–angiotensin and sympathetic nervous systems (1,4). This triggers oxidative stress in the brain, increasing catecholamine levels that deplete norepinephrine storage, reduce adrenergic receptor expression, and impair norepinephrine reuptake (4). The resulting sympathetic synapse dysfunction leads to excessive catecholamine levels, eventually becoming cytotoxic.
Myocardial Ischemia or Infarction
Cardiac sympathetic nerves are highly susceptible to ischemia and can undergo denervation after chronic ischemia or infarction (1,3,6,24). Denervation occurs at and around the infarct site, alongside ventricular remodeling (3). Similar to heart failure, neurohormonal changes lead to elevated catecholamine levels, depleted presynaptic norepinephrine storage, reduced norepinephrine transporter activity, and decreased postsynaptic adrenergic receptor expression, resulting in dysfunctional sympathetic synapses (3).
IMAGING WITH 123I-METAIODOBENZYLGUANIDINE (MIBG)
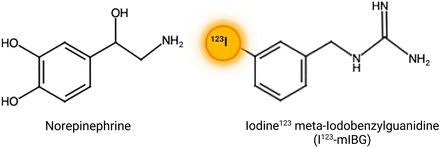
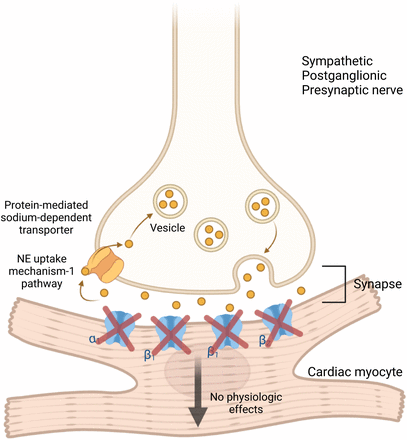
MIBG is a guanethidine analog taken up by sympathetic adrenergic neurons and chromaffin cells (6,25). Labeled with 123I, a pure γ-emitter, it can be imaged using γ-cameras. Structurally similar to norepinephrine (Fig. 3), 123I-MIBG shares its release, reuptake, and storage mechanisms but has no physiologic effect on postsynaptic receptors (Fig. 4). 123I-MIBG was initially developed in the early 1970s to image the adrenal medulla and later to detect pheochromocytoma, neuroendocrine tumors, neuroblastoma, and, to a lesser extent, carcinoid, medullary thyroid carcinoma, and paraganglioma (25–27). Normally, it is distributed in sympathetic-innervated areas, including the salivary glands, thyroid, heart, liver, spleen, adrenal glands, kidneys, and bladder (28). Stored in presynaptic sympathetic vesicles without being catabolized, 123I-MIBG uptake in the heart should be significantly higher than in the mediastinum (Fig. 4) (29). Reduced uptake indicates sympathetic denervation or dysfunction; these conditions also accelerate its washout (Fig. 5). Thus, 123I-MIBG imaging quantifies cardiac sympathetic dysfunction by measuring decreased uptake and increased washout.
Chemical structure of norepinephrine and norepinephrine radiolabeled with 123I-MIBG.
Sympathetic postganglionic presynaptic nerve synapsing with cardiac myocyte. 123I-MIBG uptake at synapse mimics norepinephrine (NE) reuptake and does not bind to adrenergic receptors on myocyte membrane nor result in downstream sympathetic effects.
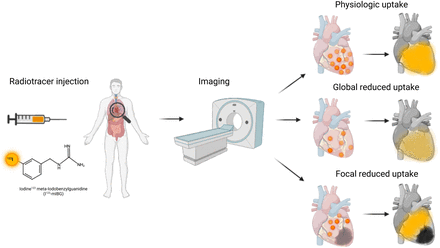
After injection of 123I-MIBG (yellow), patient undergoes imaging with 3 generalized outcomes. Normal physiologic uptake signifies global radiotracer distribution throughout heart’s sympathetic regions significantly more than background mediastinal activity. Globally decreased (lighter yellow) or focally absent uptake (black) signifies conditions with abnormally reduced sympathetic innervation, such as PD or heart failure. Lastly, focally decreased uptake can be observed in conditions causing localized defects in sympathetic innervation, such as ischemic infarcts.
Cardiac 123I-MIBG Scanning
Significant protocol and image analysis variation in the literature limits widespread clinical applicability of cardiac 123I-MIBG imaging (30,31). Standardization of 123I-MIBG imaging and analysis is required for comparison of results across different institutions, interreader reliability, ordering provider confidence in the results, and insurance reimbursement. Studies on cardiac 123I-MIBG imaging demonstrate variability in the imaging technique (planar/SPECT), collimators (low-energy, high resolution/medium energy), planar image projections (anterior/left anterior oblique), image timing (varying early and delayed time points), and analytic measurements (semiquantitative SPECT values, heart-to-mediastinum ratios [HMR], and washout rates [WRs]), among other parameters (3,30,31). The most widely used protocol in the literature is similar to that proposed by the European Association of Nuclear Medicine (EANM) Cardiovascular Committee and the European Council of Nuclear Cardiology in 2010 (30). The large, multicenter, prospective AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF) trial that used cardiac 123I-MIBG imaging for risk assessment in heart failure patients used a similar protocol with planar and SPECT imaging (32). Primarily using the EANM proposal and methods from the ADMIRE-HF trial, we share the imaging protocol used clinically in our institution with an attempt to offer a simplified procedure and image interpretation for 123I-MIBG scans (30,32–35).
Indications and Contraindications
Cardiac 123I-MIBG imaging evaluates sympathetic innervation, aiding in diagnosing and assessing cardiac dysautonomia causes such as systemic dysautonomia, amyloidosis, diabetes, Parkinsonian syndromes, heart failure, and ischemic heart disease (Table 1) (3). The scan is contraindicated in patients with known hypersensitivity to MIBG/MIBG sulfate (Table 1). Its safety in neonates under 1 mo is unestablished, and the benzyl alcohol in 123I-MIBG preparations poses additional risks in neonates, including gasping syndrome, hypotension, metabolic acidosis, and kernicterus (35). Patients with severe renal impairment or those on dialysis require special consideration, as 123I-MIBG is renally excreted but not cleared by dialysis (30). This can lead to prolonged radiation exposure and increased background activity, potentially impairing scan quality and diagnostic accuracy.
123I-MIBG Cardiac Scintigraphy Provider Summary and Protocol
Patient Information and Preparation
Patients may have concerns about radiation exposure and should receive written instructions covering the procedure, benefits, risks, preparation, and postscan guidelines (Table 1). Reassurance on radiation exposure to others and methods to minimize it for themselves should be provided. The following patient preparation items, detailed in Tables 1 and 2, are particularly pertinent:
Common Drugs Affecting 123I-MIBG Uptake
Certain medications interfere with 123I-MIBG uptake and may need to be withheld, whereas essential cardiac medications may be continued if necessary (Table 2) (29,30,36–38).
Foods containing vanillin or catecholaminelike compounds (e.g., chocolate, blue cheese) should be avoided before the scan (39).
Pregnancy and lactation should be assessed, as 123I crosses the placenta and is excreted in breast milk. Breastfeeding should be discontinued for at least 48 h, with the package insert suggesting up to 6 d to minimize risk (30,35).
To protect the thyroid, iodine or perchlorate medications should be administered before the scan (36,40,41).
Patients with a prior thyroidectomy do not need thyroid blocking.
Hydration and frequent voiding help reduce bladder radiation exposure, as 123I-MIBG is excreted primarily through the kidneys and is not cleared by dialysis (30). Patients with severe renal impairment require special consideration because of prolonged radiation exposure.
Radiopharmaceutical Administration
An activity of 10 mCi (370 MBq) of 123I-MIBG should be administered via standard slow intravenous injection over 1–2 min for patients 16 y or older or for patients weighing 70 kg or more if less than 16 y of age (Table 1) (30,35,37). For pediatric patients younger than 16 y and weighing less than 70 kg, the North American Consensus Guidelines’ recommended activity is 0.14 mCi/kg (5.2 MBq/kg) with a minimum recommended activity of 1 mCi (37 MBq), whereas the EANM pediatric guidelines are slightly higher (42,43). The radiopharmaceutical injection should be followed by an injection of 0.9% sodium chloride to ensure complete radiopharmaceutical delivery.
Image Acquisition
Assessment of uptake can be obtained with early (10–15 min) and delayed (3 h 50 min or 4 h) anterior planar imaging for 10 min each (Table 3) (29,30,32–34). The delayed time point not only incorporates neuronal integrity seen in the early images but also incorporates the complete neuronal functioning of norepinephrine uptake, storage in vesicles, and release and reuptake mechanism. At our institution, because of the delayed image advantages and consistent results, we only perform delayed imaging.
123I-MIBG Scintigraphy Imaging Parameters
A γ-camera with a large field of view should be used. The collimators should be low-energy, high-resolution, parallel hole collimators (Table 3). Energy windows should be centrally set at 20%, with the window centered at a 159-keV photopeak. A preferable planar image matrix of 256 × 256 is used if possible, but a 128 × 128 matrix may be used if a higher resolution is not available (29).
The patient should be in the supine position, with female patients imaged without a bra. SPECT with or without CT should be performed for adequate visual assessment and to avoid any confounding 123I-MIBG activity on anterior planar images. SPECT images also eliminate the need for lateral planar images. SPECT images are performed with a patient’s arms elevated above the head.
Image Processing and Quantification
Cardiac 123I-MIBG uptake is assessed using the HMR, calculated from counts per pixel in regions of interest (ROI). Although both early and delayed images can be used, delayed HMR is preferred because of reduced confounding from blood pool uptake and more consistent results. This approach aligns with the ADMIRE-HF trial, which used delayed HMR for primary analysis (32). Normal delayed uptake indicates intact postganglionic sympathetic innervation (44).
For HMR calculation, the anterior projection is used, with an ROI drawn over the heart. Some protocols contour the myocardium precisely, whereas others encompass the entire heart to reduce variability (45). This method also simplifies the process for technologists, particularly in cases in which low or absent uptake makes delineation of the ventricular wall challenging on planar images. In our practice, we typically use a circular ROI encompassing the entire heart or a freehand ROI to match its shape, calculating mean counts per pixel.
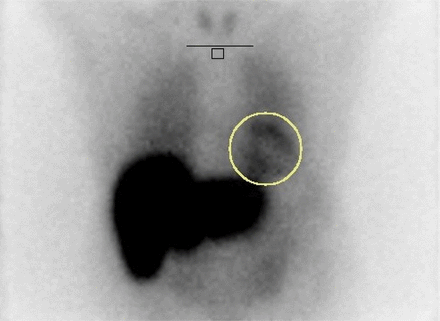
For the mediastinum, conventional 7 × 7 pixel ROI placement on a 128 × 128 matrix can be cumbersome because of patient variability and software limitations (35,37). Instead, we use a longitudinal rectangular ROI in the midline mediastinum at least 4 pixels below the clavicular heads, avoiding the thyroid, muscles, lungs, or abnormal MIBG activity (Fig. 6). This ensures placement in the area with the lowest background counts, from which the mean mediastinal counts per pixel are calculated. The HMR is then determined by dividing mean myocardial counts by mean mediastinal counts, providing a key semiquantitative measure of myocardial sympathetic innervation:
Example of 123I-MIBG ROIs drawn for HMR calculation. Horizontal black line indicates location of clavicular heads, below which there is freehand rectangle for mediastinal background ROI (our technologists freehand draw these rectangles). Yellow circle encloses myocardial uptake.
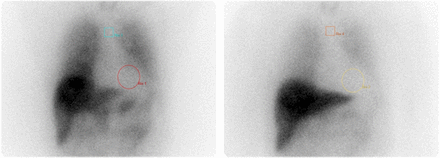
Myocardial WR reflects nerve integrity and sympathetic drive, mainly representing the norepinephrine uptake-1 mechanism (44). Cardiac disorders increase sympathetic activity and decrease norepinephrine reuptake, causing higher 123I-MIBG washout and decreased delayed cardiac uptake. The WR is calculated as the percentage decrease in myocardial radiotracer from early to delayed images, normalized to mediastinal activity (Fig. 7):
Example of early (15 min) (A) and delayed (4 h) (B) images for 123I-MIBG scan demonstrating abnormal increased WR of 42.45%.
SPECT Reconstruction
SPECT or SPECT/CT imaging improves contrast resolution and should be performed to better visualize uptake in the myocardium (3,31,46). Additionally, SPECT imaging can provide segmental analysis of 123I-MIBG uptake throughout the left ventricle. Segmental analyses in the literature use a semiquantitative technique on 5–20 segments, commonly scoring 0–3 or 0–4 for each segment (0 represents normal uptake and 3 or 4 represent absent uptake) (47,48). These scores can be summed for the left ventricle and correlated with disease pathology. For example, a summed score of 26 or greater on delayed SPECT imaging was shown to predict implantable cardioverter–defibrillator therapy appropriateness and cardiac death among heart failure patients (3). Further work on SPECT-delayed summed-score cutoffs needs to be conducted for a variety of cardiac pathologies to determine the optimal prognostic scores.
For SPECT imaging, we use a dual head camera, 128 × 128 matrix with a 360° orbit and 180° acquisition starting at 45° right anterior oblique and proceeds counterclockwise to 45° left posterior oblique. We use 120 steps (60 steps per detector) and 30 s per step. No gating is performed.
INTERPRETATION
123I-MIBG interpretation includes qualitative, semiquantitative, and quantitative methods. Planar imaging uses qualitative and quantitative analysis, whereas SPECT imaging uses semiquantitative techniques. Early (15 min) and delayed (4 h) imaging should be evaluated qualitatively for myocardial uptake relative to the mediastinum and quantitatively for the HMR and WR. Normal delayed HMR ranges from 1.9 to 3.0, whereas WR varies between 21% and 37%, though values depend on physiologic and technical factors (31,49,50). Age, sex, cardiac function, and activity level impact physiologic variability, with HMR decreasing and WR increasing with age (3). Technical factors include ROI placement, administered activity, 123I-MIBG–specific activity, and image acquisition settings (e.g., collimation, acquisition time) (3,5,31,51).
SPECT imaging uses a summed segmental approach, similar to perfusion imaging, but regional uptake differences create interpretation challenges, especially in older patients and men (3,31,48,52). Athletes may show lower inferior wall uptake due to increased vagal tone (53). Additionally, attenuation artifacts and absent myocardial uptake can affect SPECT accuracy (5,31).
Despite these variations, 123I-MIBG remains a valuable tool for assessing sympathetic denervation and cardiac dysautonomia across multiple pathologies (3,5,31,54). Our institution has established HMR and WR thresholds based on data from the largest studies in the literature (Table 4) (32,46,48,55–60).
Large Clinical Trials and Cohort Studies Using 123I-MIBG for Heart Failure Prognostication and PD or DLB Diagnosis
CHARACTERIZING CARDIAC AUTONOMIC DYSFUNCTION WITH 123I-MIBG
Disruption of cardiac neural networks through intrinsic and extrinsic mechanisms results in autonomy dysfunction, which can be characterized with 123I-MIBG imaging. We will now discuss studies that demonstrate the utility of 123I-MIBG scans in the previously described disorders.
Intrinsic Mechanisms
Dysautonomia
Among the causes of cardiac dysautonomia, 123I-MIBG imaging has been used to assess cardiac dysautonomia in postural orthostatic tachycardia syndrome (POTS). Haensch et al. studied 20 patients with POTS using 123I-MIBG SPECT imaging, comparing HMR with a normal threshold of more than 1.70 (61). Four patients with POTS showed significantly reduced uptake (mean HMR, 1.22 ± 0.08) compared with control patients with incidental tumors (mean HMR, 1.86 ± 0.18). The authors noted limitations, including the young age of participants, lack of age-matched controls, and variability in sympathetic cardiac denervation among POTS patients (61).
Amyloidosis
123I-MIBG imaging detects sympathetic denervation in cardiac amyloidosis, showing reduced HMR values below 1.60 at 4 h (13). Lower HMR predicts higher mortality, with a 42% 5-y all-cause mortality rate for values under 1.60, compared with 7% for those 1.60 or greater (13,62). All forms of cardiac amyloidosis (immunoglobulin light chain amyloidosis, hereditary ATTR, and wild-type ATTR) show reduced HMR (13,14). Although 123I-MIBG cannot distinguish amyloid subtypes unlike amyloid PET imaging or bone scintigraphy, studies suggest it may detect cardiac involvement earlier than 99mTc-PYP can (12,63). In a study of 75 hereditary ATTR patients, 65% had cardiac denervation on 123I-MIBG (HMR < 1.85), whereas only 39% showed abnormal 99mTc-PYP uptake (63). Notably, 29% of 99mTc-PYP–negative patients had abnormal 123I-MIBG HMR, correlating with echocardiogram results, indicating that 123I-MIBG may detect early cardiac involvement.
Diabetes Mellitus
Diabetes commonly causes cardiac dysautonomia because of sympathetic nerve damage, detectable with 123I-MIBG imaging, especially using SPECT and left ventricular segmental analysis (3,18,19). Langer et al. compared 123I-MIBG and 99mTc-sestamibi scans in 65 asymptomatic diabetic patients and 23 controls, assessing segmental uptake (0 = normal to 4 = no uptake) and clinical autonomic dysfunction (19). Diabetic patients showed reduced 123I-MIBG uptake in all segments except the septum compared with controls, with more severe reductions in those with clinical dysautonomia. Silent ischemic areas also exhibited greater uptake reduction because of neuronal ischemia. Nagamachi et al. studied 144 patients with type 2 diabetes and found that an HMR less than 1.7 independently predicted long-term mortality and, when combined with documented autonomic neuropathy, predicted cardiac-related death (18). Although 123I-MIBG imaging shows diagnostic and prognostic promise in diabetes, further research is needed.
Parkinsonian Syndromes
PD causes degeneration of postganglionic sympathetic cardiac nerves, leading to cardiac dysautonomia detectable with 123I-MIBG imaging (22,23,64). Japanese researchers first observed reduced HMR in PD patients (65–67). Satoh et al. found significantly lower HMR (<1.50) and higher WRs (>0.4) in 35 patients with PD compared with 24 controls (HMR, ∼2.0; WR, <0.35), also confirming that anti-PD medications do not improve 123I-MIBG uptake (66).
Unlike PD, MSA and progressive supranuclear palsy preserve postganglionic cardiac innervation (23,64,68), Sakuramoto et al. demonstrated 100% and 90% specificity in distinguishing PD from MSA and progressive supranuclear palsy using 123I-MIBG (23). Eckhardt et al. also found significantly lower HMR in PD (1.18) than in MSA (1.79), making 123I-MIBG a valuable tool for differentiating Parkinsonian syndromes with similar neurologic symptoms (68).
DLB, another Parkinsonian syndrome, also shows reduced cardiac 123I-MIBG uptake, distinguishing it from Alzheimer dementia (69–71). Watanabe et al. found a median HMR of 1.2 in patients with DLB versus 2.4 in patients with Alzheimer and in controls (71). Manabe et al. further confirmed significantly lower HMR in DLB compared with non-DLB dementias, with an area under the curve of 0.879 for a 2.23 HMR cutoff, reinforcing 123I-MIBG as a diagnostic tool for differentiating DLB from other dementias (70). These results demonstrate the utility of 123I-MIBG scans as an additional tool for diagnostic differentiation of DLB from other dementias.
Extrinsic Mechanisms
Heart Failure
Chronic left ventricular heart failure leads to reduced presynaptic 123I-MIBG uptake and increased washout, providing valuable prognostic and management insights (2,5,29,32,47,50,72). Merlet et al. studied 112 patients with heart failure (New York Heart Association class II–IV, left ventricular ejection fraction < 40%) over a mean of 27 mo, identifying low HMR and left ventricular ejection fraction as independent mortality predictors (72). Patients had an average delayed HMR of 1.23 ± 0.19, compared with a normal HMR of 1.95 ± 0.31. Nakata et al. followed 414 patients (42% with symptomatic heart failure) for an average of 22 mo, finding that an HMR of no more than 1.74 independently predicted cardiac death due to heart failure, sudden cardiac death, or myocardial infarction (59). The ADMIRE-HF study (961 patients, New York Heart Association class II/III, left ventricular ejection fraction ≤ 35%) confirmed that an HMR of less than 1.60 was linked to higher mortality and cardiac event rates over 2 y, with a hazard ratio of 0.36 (32). A pooled analysis of 1,322 patients with heart failure identified an HMR of 1.68 or less and a WR greater than 43% as predictors of lower overall survival in New York Heart Association class I–IV patients (60). Patients were also categorized into low-risk (>2.10), intermediate-risk (1.40–2.10), and high risk (<1.40) groups, with delayed HMR independently predicting all-cause mortality (hazard ratio, 0.85). 123I-MIBG imaging has also demonstrated treatment benefits, showing improved uptake and HMR ratios with β-blockers, angiotensin-converting enzyme inhibitors, amiodarone, left ventricular assist devices, and cardiac resynchronization therapy (3). These findings support the role of 123I-MIBG in prognosis and heart failure management.
Myocardial Ischemia or Infarction
Myocardial ischemia and infarction damage both postsynaptic myocytes and postganglionic sympathetic neurons, detectable with 123I-MIBG imaging (3). Tomoda et al. compared 123I-MIBG and 201TlCl imaging in 8 patients with myocardial infarction and 12 with unstable angina, finding 123I-MIBG uptake defects in all patients with myocardial infarction (8/8) and in 7 of 12 patients with angina, whereas 201TlCl defects were present in only 4 of 8 patients with myocardial infarction and none of the patients with angina (73). This suggests 123I-MIBG may be more sensitive for ischemia detection than older perfusion agents. In 30 asymptomatic individuals with a strong family history of early coronary artery disease, reduced delayed 123I-MIBG uptake correlated with coronary artery stenosis despite normal 99mTc-sestamibi perfusion (74). Additionally, 123I-MIBG defects align with left ventricular remodeling postmyocardial infarction, reinforcing its diagnostic and prognostic role in ischemic heart disease, even in asymptomatic patients (75).
CONCLUSION
123I-MIBG imaging is a valuable tool for assessing cardiac sympathetic innervation in conditions such as heart failure, ischemic heart disease, PD, and DLB. However, the lack of standardized protocols has led to variability in image acquisition and interpretation across institutions, limiting universally accepted benchmarks. To improve consistency, we propose a simplified protocol based on EANM guidelines and the ADMIRE-HF trial, standardizing patient preparation, image acquisition, and interpretation using metrics such as HMR. A uniform approach will enhance diagnostic accuracy and improve the management of cardiac sympathetic dysfunction.
DISCLOSURE
The views expressed in this article are those of the authors and do not reflect the official policy of the Uniformed Services University, Department of Army/Navy/Air Force, Department of Defense, or U.S. Government. The authors used ChatGPT-4o (OpenAI, 2024) to provide editorial suggestions and text refinement for clarity and conciseness during the revision process. All final content, interpretations, and conclusions are the responsibility of the authors. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can a standardized protocol for cardiac 123I-MIBG imaging be established to improve the detection, characterization, and prognostic assessment of sympathetic dysfunction across various cardiac conditions?
PERTINENT FINDINGS: Cardiac sympathetic dysfunction arises from intrinsic (e.g., dysautonomias, amyloidosis, Parkinsonian syndromes) and extrinsic (e.g., heart failure, myocardial ischemia) mechanisms. Standardized 123I-MIBG imaging protocols, incorporating qualitative, semiquantitative, and quantitative methods (e.g., HMR and WR), can accurately assess disease severity and predict outcomes. Variability in image acquisition and interpretation has limited universal benchmarks, highlighting the need for a simplified and consistent imaging approach.
IMPLICATIONS FOR PATIENT CARE: Implementing a standardized cardiac 123I-MIBG protocol will improve diagnostic accuracy, enhance prognostic evaluation, and ensure consistency across institutions. This will lead to better clinical decision-making, optimized treatment strategies, and improved patient outcomes in those with cardiac autonomic dysfunction.
ACKNOWLEDGMENTS
We thank Prince Anbiah, CNMT, CT(NM), at Walter Reed National Military Medical Center for his help in figure generation. We want to thank Mary Beth Farrell, CNMT, at Molecular Imaging Services and Aspen University for her helpful edits to the article.
Footnotes
*Contributed equally to this work.
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. You may make 3 attempts to pass the test and must answer 80% of the questions correctly to receive credit—number of credits awarded will be determined by the length of the article. Participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through June 2028. Additional details such as the number of credits issued per article, expiration dates, financial disclosure information, VOICE transcripts, and the process to earn CE credit can also be found in the SNMMI Learning Center.
Published online Apr. 22, 2025.
REFERENCES
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.
- 49.
- 50.
- 51.
- 52.
- 53.
- 54.
- 55.
- 56.
- 57.
- 58.
- 59.
- 60.
- 61.
- 62.
- 63.
- 64.
- 65.
- 66.
- 67.
- 68.
- 69.
- 70.
- 71.
- 72.
- 73.
- 74.
- 75.
- Received for publication December 27, 2024.
- Accepted for publication March 18, 2025.