Abstract
Annexin A5 is a phospholipid binding protein with high affinity for phosphatidyl-serine, which is externalized by cells undergoing programmed cell death. An increased programmed cell death rate has been reported in the heart after myocardial infarction (MI). The aim of this study was to correctly localize annexin A5 uptake in vivo and to determine the area at risk in humans with acute MI. Methods: Nine patients were studied. Before reperfusion was achieved, 99mTc-sestamibi was injected intravenously. Myocardial 99mTc-sestamibi perfusion scintigraphy was performed after reperfusion. Thereafter, 99mTc-labeled annexin A5 was administered intravenously, followed by scintigraphic imaging of the heart. Myocardial 99mTc-sestamibi scintigraphy was repeated 1–3 wk after the MI onset. 99mTc-Annexin uptake was also studied in the subacute phase of the MI in 2 patients. Results: All patients clearly showed perfusion defects on 99mTc-sestamibi scintigraphy in concordance with the infarct location. Furthermore, all patients showed accumulation of 99mTc-annexin A5 at the infarct site, indicating that cardiomyocytes with externalized phosphatidyl-serine are present in the infarct area. 99mTc-sestamibi defects determined 1–3 wk after the MI onset were significantly smaller than the defects in the acute phase. 99mTc-annexin uptake was absent in the 2 patients studied in the subacute phase. Conclusion: In acute MI, an increase of programmed cell death can be correctly localized in vivo in the area at risk. Furthermore, the decrease in 99mTc-sestamibi defect size in the subacute phase of the MI further suggests that in parts of the area at risk, reversible myocardial damage rather than necrosis is present in cardiomyocytes.
Previous investigations have demonstrated the presence of programmed cell death (PCD) in the hearts of animals (1–4) and humans (5,6) after acute myocardial infarction (MI), by investigating the heart at necropsy. PCD was detected using annexin A5 (7), a protein that binds to cells exposing phosphatidyl-serine (PS). This PS expression is one of the first events during PCD, persisting until the final degradation of the cell (8). The naturally occurring protein annexin A5 (36 kDa) binds with high affinity to negatively charged PS on apoptotic cells. For this reason, annexin A5 has been used for both in vitro and in vivo detection of apoptosis (9,10).
Our group demonstrated that cell death in human hearts can be monitored noninvasively using 99mTc-(n-1-imino-4-mercaptobutyl)-annexin A5 (i-anxA5) (11). Reconstruction of oblique images as commonly done in cardiac perfusion studies was difficult because only focal uptake in a small region of the heart was observed, making it difficult to exactly localize the area.
Recently, 99mTc-4,5-bis(thioacetamido)pentanoyl-annexin A5 (BTAP-anxA5) (Apomate; Theseus Imaging Corp., Cambridge, MA) became available for the detection of human cell death.
The aim of the present study was to determine the area at risk in the acute phase of MI using 99mTc-sestamibi (MIBI) and to localize uptake of 99mTc-annexin A5 in vivo in the area at risk using 99mTc-BTAP-anxA5. 99mTc-annexin A5 activity was also studied in the subacute phase of MI in 2 patients. Furthermore, a quantification program was used to determine the size of the 99mTc-MIBI defect, both in the acute and in the subacute phase of MI.
MATERIALS AND METHODS
Nine patients (6 male, 3 female; mean age, 61 y; range, 42–77 y) with primary acute MI who presented within 6 h of the onset of symptoms at the cardiovascular emergency room of our hospital were included in this study. Figure 1 shows the time line of the study protocol. The diagnosis and localization of an acute MI was made by electrocardiographic criteria and confirmed by biochemical detection of cardiac enzyme release. All patients underwent percutaneous transluminal coronary angioplasty (PTCA) of the infarct-related vessel, 4 as primary PTCA and 5 as rescue PTCA after thrombolytic therapy had failed. All PTCA procedures resulted in level III flow, according to the classification of the Thrombolysis in Myocardial Infarction trial. Written informed consent was obtained from all patients. The study was approved by the medical ethics committee of the University Hospital Maastricht, The Netherlands.
Time line of study protocol. Numbers of patients studied are in parentheses.
Immediately after the diagnosis of acute MI was confirmed, but before vascularization was restored, a mean dose of 260 MBq (range, 200–300 MBq) 99mTc-MIBI (Cardiolite; Du Pont Pharma, Brussels, Belgium) was administered in 8 of the 9 patients (excluding the first patient). MIBI-SPECT was performed after reperfusion (approximately 2.5 h later; range, 50–300 min) to measure the size of the perfusion defect in the acute phase, reflecting the area at risk in the heart. Immediately after MIBI-SPECT, 99mTc-annexin A5 was injected. 99mTc-BTAP-anxA5 was labeled with pertechnetate to 99mTc-BTAP-anxA5 according to the manufacturer’s procedure (Theseus Imaging Corp.) as described elsewhere (12). In short, an ester ligand of 99mTc and phenthioate was produced. After incubation of the 99mTc-ester ligand with annexin A5, 99mTc-BTAP-anxA5 was formed and subsequently purified using column chromatography. Radiochemical purity was 97% (SD, 2%). For scintigraphy, a mean of 410 MBq (range, 200–500 MBq) 99mTc-BTAP-anxA5, corresponding to approximately 800 μg annexin A5, was given intravenously after reperfusion had been achieved. Fifteen and 20 h after the injection of 99mTc-annexin A5, SPECT was performed. All SPECT studies were done with a MultiSPECT 2 dual-head gamma camera (Siemens, Hoffman Estates, IL) using low-energy high-resolution collimators, an energy peak of 140 keV with a 15% window, a 64 × 64 matrix, a zoom factor of 1.45, and 60 angle views of 45 s each. Studies were reconstructed with a filtered backprojection method, using a Butterworth filter with a cutoff frequency of 0.55 and an order of 5.
In 2 patients, the 99mTc-annexin A5 study was repeated in the subacute phase as part of another, recently started study protocol. In 1 patient 3 mo after the MI onset, and in the other patient 4 and 8 d after the MI onset, SPECT was performed 15 h after the infusion of the radiopharmaceutical.
Five to 19 d after the onset of the MI, 8 patients underwent routine myocardial stress-rest scintigraphy, 7 by means of 99mTc-labeled MIBI, 1 by means of 201Tl to rule out cardiac ischemia. Furthermore, echocardiography was performed on 6 patients within the first 2 wk after the MI onset (1 patient was not studied because of withdrawal from follow-up). Quantification of the myocardial 99mTc-MIBI activity area was performed by using a program as described elsewhere (13,14). In short, the raw data are reconstructed and reformatted to short-axis slices as conventionally is done in myocardial perfusion studies. Our program extracts radial long-axis slices from these data, which are used for semiquantitative analysis and comparison with a normal database. In the extraction of the radial long-axis slices, tools are available to improve centering and alignment of the long axis of the heart within the frame of analysis. Data on 99mTc-MIBI defect size were analyzed by the Student t test. A P value less than 0.05 was considered statistically significant.
RESULTS
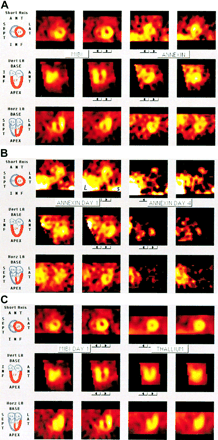
Patient characteristics are shown in Table 1. All patients showed decreased uptake of 99mTc-MIBI in an area corresponding to the location of the infarct as diagnosed on electrocardiography. In the same photopenic area, an increased uptake of 99mTc-annexin A5 was seen in all patients, indicating the presence of cardiomyocytes with externalized PS in the infarct area. Best images were obtained 15 h after the infusion, showing 99mTc-annexin A5 activity at the infarct site and little 99mTc-MIBI rest activity (“MIBI ghost”). SPECT reconstruction appeared to be difficult in the acquisitions 20 h after the injection because of advanced decay of 99mTc-MIBI and 99mTc-annexin A5 activity. Figure 2A shows the area at risk on the reconstructed 99mTc-MIBI pictures of a patient with an acute anteroseptal MI. Figure 2B shows 99mTc-annexin A5 uptake in the same patient. Examples of decreased MIBI and increased 99mTc-annexin A5 activity for the same patient are shown in Figure 2C and for a patient with an inferior wall infarction in Figure 2D. Figure 2C clearly shows the decreased 99mTc-MIBI activity at the anteroseptal region of the myocardium and the increased 99mTc-annexin A5 uptake at the same site of the myocardium. Figure 2D shows the inferior 99mTc-MIBI defect and the 99mTc-annexin A5 uptake at the same location. All other patients showed consistently comparable results.
(A) Area at risk: 99mTc-MIBI injected before reperfusion. Reconstructed myocardial images of patient 2 after injection of 99mTc-MIBI in cardiovascular emergency room. Short-axis images show anteroseptal defect; vertical long-axis images, anteroapical defect; and horizontal long-axis images, apicoseptal defect. (B) Detection of cell death: 99mTc-annexin A5 after reperfusion. Reconstructed myocardial images of patient 2, 15 h after injection of 99mTc-BTAP-anxA5. Short-axis images show increased activity anteroseptally; vertical long-axis images, anteroapical hot spot; and horizontal long-axis images, increased apicoseptal activity. (C) 99mTc-annexin A5 uptake in area at risk: combination of parts A and B. Left 2 columns show 99mTc-MIBI activity; right 2 columns, 99mTc-annexin A5. (D) Acute inferior MI of patient 5: combination of some reconstructed images of 99mTc-MIBI activity (left 2 columns) and 99mTc-annexin A5. ANT = anterior; INF = inferior; LA = long axis or left atrium; LAT = lateral; LV = left ventricle; RA = right atrium; RV = right ventricle; SEPT = septal; Vert = vertical; Horz = horizontal.
Patient Characteristics and Infarct Location
99mTc-MIBI imaging 5–19 d after onset of the MI (n = 7) still showed a defect, although much smaller, corresponding to the area of increased 99mTc-annexin A5 uptake and no signs of ischemia.
Echocardiography was performed on 6 patients, and 5 patients showed decreased wall motion corresponding to the diagnosed infarct localization obtained by angiography (Table 1).
Quantification on 99mTc-MIBI SPECT pictures revealed defect areas ranging from 9% to 43% of the left ventricle, defining the area at risk in the acute phase, whereas 99mTc-MIBI defects ranged from 3% to 25% of the left ventricle in the subacute phase of the MI. All patients on whom 99mTc-MIBI in both the acute and the subacute phase was performed (n = 6) showed decreased 99mTc-MIBI defects (P < 0.05) on the late images when compared with the acute MIBI of 12% on average (Fig. 3).
99mTc-MIBI defects (n = 6) expressed as percentage of left ventricle both in acute phase and in subacute phase of MI.
In 2 patients, 99mTc-annexin A5 was reinjected in the subacute phase, in 1 patient 3 mo after the MI onset, and in the other patient 4 d and also 8 d after the MI onset. In the latter patient, 99mTc-annexin A5 uptake was reduced on the 4-d images (Figs. 4A and 4B) when compared with the initial images. After 8 d, no 99mTc-annexin A5 activity was present at the infarct site. Furthermore, 201Tl scintigraphy performed 1 mo after the acute MI showed complete normalization of the affected area (Fig. 4C), indicating that the defect on the acute MIBI or the 99mTc-annexin A5 activity on day 1 (Fig. 4A) represents reversible myocardial damage. There also was no 99mTc-annexin A5 activity present in the myocardium of the patient studied 3 mo after the MI onset.
Repeated studies of 1 patient. (A) Combination of acute 99mTc-MIBI and 99mTc-annexin A5 uptake in area at risk on day 1 in patient studied several times. 99mTc-MIBI defect in anteroseptal and apical region (left 2 columns) correlates well with 99mTc-annexin A5 activity (right 2 columns). (B) 99mTc-annexin A5 activity compared on days 1 and 4 of acute MI. 99mTc-annexin A5 activity is already reduced after 4 d (right 2 columns), compared with day 1 (left 2 columns). L indicates liver activity; s indicates spleen activity. (C) 201Tl images (right 2 columns) clearly show complete recovery of area at risk seen on 99mTc-MIBI scan as performed at onset of MI (left 2 columns). ANT = anterior; INF = inferior; LA = long axis or left atrium; LAT = lateral; LV = left ventricle; RA = right atrium; RV = right ventricle; SEPT = septal; Vert = vertical; Horz = horizontal.
All patients recovered well after treatment in the coronary care unit. No side effects were observed after the injection of either 99mTc-MIBI or 99mTc-annexin A5.
DISCUSSION
Apoptosis or PCD is known to occur during physiologic as well as pathologic processes in the human body, such as growth and inflammatory events on the one hand and Alzheimer’s disease or MI on the other hand. With the latter, both cardiomyocyte dysfunction and irreversible cell loss might occur, which can lead to the decline in left ventricular contraction, one of the factors related to earlier death (15). Better understanding of the cellular apoptotic pathway may lead to the development of new therapeutic drugs that might limit this cardiomyocyte loss.
Most previous investigations have been performed either in vitro or in animal studies. Flow cytometry (16) or terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (17) was commonly used to identify apoptotic cells in vitro. Animal studies have shown that increased 99mTc-annexin A5 uptake in transplanted hearts of rats correlated well with the in vitro detection of apoptosis using annexin A5 immunofluorescence microscopy (18). Animal studies also indicate that ischemia of the heart, followed by reperfusion, induces reperfusion injury with a substantial loss of cardiomyocytes through apoptosis (4). The feasibility of an in vivo approach in humans with acute MI after reperfusion was recently published by our group using 99mTc-i-anxA5 (11). In 6 of 7 patients investigated, an increased uptake of 99mTc-i-anxA5 was seen in the infarct area of the heart on early and late SPECT images. No uptake was observed in the heart of a healthy volunteer or outside the infarct area as seen in MIBI studies performed in the subacute phase of the MI. This study showed that PCD can indeed be visualized in patients with acute MI. The next step was to determine the area at risk and the 99mTc-annexin A5 activity (PCD) in this area.
In the present study, in which combined 99mTc-MIBI and 99mTc-annexin A5 images were generated for a similar group of patients, the area at risk could be well defined on the MIBI pictures. Because of the storage of 99mTc-MIBI in the mitochondrion of cardiomyocytes (and lack of redistribution), imaging could be postponed until after revascularization was achieved, which is advantageous to the use of 201Tl-chloride for the detection of cardiovascular perfusion defects. Furthermore, a MIBI image of the heart could still be seen vaguely (“99mTc-MIBI ghost-image”) in the unaffected myocardial area on the 99mTc-annexin A5 scintigraphy. This technique allowed better SPECT reconstruction and much better localization of the 99mTc-annexin A5 activity in the heart. Using this protocol, it was indeed quite easy to correctly localize 99mTc-annexin A5 activity in all patients at the myocardial region corresponding to the 99mTc-MIBI defect. We chose to obtain images 15 and 20 h after the injection of 99mTc-annexin A5 on the basis of observations from the previous study (11). From the present study, we learned that best images were obtained 15 h after the infusion. Recent studies indicate that imaging earlier after injection is feasible (P.W.L. Thimister, unpublished data, 2002). Because scintigraphic results were in concordance with angiography and ultrasound results, the protocol used in our study could soon be well suited for evaluation of new antiapoptotic drug treatment.
Along with apoptotic characteristics, necrotic cell death characteristics were reported to be present in the morphology of annexin A5-positive cardiomyocytes (4,19,20), suggesting that part of the cardiomyocytes may not show the classic morphologic characteristics of apoptosis. So the question remains whether enhanced 99mTc-annexin A5 uptake in the heart is caused by an ongoing cell-death program or whether it is more the expression of the presence of necrotic cells as can be seen in the final stage of cell death (15,21,22). The decreased 99mTc-MIBI defect in the subacute phase, when compared with the acute phase of the MI, strongly suggests that at least part of the myocardial 99mTc-annexin A5 activity as present in the acute phase represents potentially reversible myocardial cell damage rather than necrosis. A direct proof for the presence of apoptosis would require cardiac biopsy samples showing DNA fragmentation, and obtaining such samples in our patient group was ethically not acceptable.
Another interesting observation in this study was the decreased 99mTc-annexin A5 activity in a patient who was repeatedly studied 3 and 8 d after the MI onset. Because no activity remained after 8 d, regeneration of myocardial cells might have taken place, in which case PS expression is no longer present. This would imply either that the ischemic part of the MI has been restored to viable tissue or that cells have been removed as necrotic material.
The main clinical utility of this in vivo detection of cell death with 99mTc-annexin A5 scintigraphy would be the evaluation of new therapeutic strategies that intervene in myocardial cell death, the so-called cell death inhibitors. It is this noninvasive in vivo detection technique that will make it possible to test the therapeutic potentials of these substances. The application of this methodology may be extended to any situation of progressive myocardial dysfunction. This study also implies that performing 99mTc-annexin A5 scintigraphy will soon be possible to provide more information about the kinetics of the cell death process in different clinical forms of myocardial disease. This characterization of disease might affect clinical decision making.
CONCLUSION
This study, together with earlier animal and human studies, indicates that PCD (apoptosis) can be detected in vivo at the infarct area. Furthermore, with this study protocol, the area at risk can be well defined. Also, the decrease in 99mTc-MIBI defect size in the subacute phase of the MI further suggests that in parts of the area at risk, reversible myocardial damage rather than necrosis is present in cardiomyocytes.
Acknowledgments
We thank Marie-Thérèse Pakbiers and Denise Janssen for expert technical assistance and Theseus Imaging Corp. for the generous supply of 99mTc-BTAP-anxA5.
Footnotes
Received Jan. 15, 2002; revision accepted Jul. 9, 2002.
For correspondence or reprints contact: Paul W.L. Thimister, MD, PhD, Department of Nuclear Medicine, Maasland Hospital Sittard, Walramstreet 23, Sittard, 6131 BK, The Netherlands.
E-mail: p.thimister@orbisconcern.nl