Abstract
The bone-seeking property and the potential exposure of red marrow by the α-particle emitter 223Ra (half-life, 11.43 d) were compared with those of the β-emitter 89Sr (half-life, 50.53 d). Methods: The biodistributions of 223Ra and 89Sr were studied in mice. Tissue uptake was determined at 1 h, 6 h, 1 d, 3 d, and 14 d after intravenous administration. Radiation absorbed doses were calculated for soft tissues and for bone. Multicellular-level doses were estimated for bone marrow cavities. Results: Both 89Sr and 223Ra selectively concentrated on bone surfaces relative to soft tissues. The measured bone uptake of 223Ra was slightly higher than that of 89Sr. At 24 h, the femur uptake of 223Ra was 40.1% ± 7.7% of the administered activity per gram of tissue. The uptake in spleen and most other soft tissues was higher for 223Ra than for 89Sr. Although predominant clearance of 223Ra was observed from the soft tissues within the first 24 h, the bone uptake of 223Ra, which was not significantly different from maximum after only 1 h, was not significantly reduced during the 14 d. Furthermore, little redistribution of 223Ra daughter products away from bone was found (2% at 6 h and less than 1% at 3 d). Estimates of dose to marrow cavities showed that the 223Ra α-emitter might have a marrow-sparing advantage compared with β-emitters for targeting osteoid surfaces because the short-range α-particles irradiate a significantly lower fraction of the marrow volumes. At the same time, the bone surfaces will receive a therapeutically effective radiation dose. Conclusion: The results of this study indicate that 223Ra is a promising candidate for high-linear-energy transfer α-particle irradiation of cancer cells on bone surfaces. 223Ra can, together with its daughter radionuclides, deliver an intense and highly localized radiation dose to the bone surfaces with substantially less irradiation of healthy bone marrow compared with standard bone-seeking β-emitters.
Bone-targeting radiopharmaceuticals, such as the β-emitters 32P orthophosphate (NaH2PO4) and 89Sr (as strontium chloride, Metastron; Amersham Health, Princeton, NJ), have been used extensively for relief of bone pain associated with metastatic lesions in the skeleton (1,2). Because the major dose-limiting factor with this treatment modality is toxicity to the normal bone marrow cells (3–6), the range of the β-particles from 32P (maximum energy [Emax],1.7 MeV; range, ∼6 mm) and 89Sr (Emax, 1.49 MeV; range, ∼5.5 mm) can be a disadvantage. To minimize marrow toxicity, radionuclides that emit particles of shorter range have been studied for bone pain relief. To date, clinical studies on new radionuclides have been limited mainly to the low-energy β-emitters 153Sm (7) and 186Re (8), and a conversion electron emitter, 117mSn (9).
Preclinical data on animals and radiation dose estimates indicate that α-particle radiation should be excellent for treating metastatic cancers. The short-α-particle ranges in tissue (<100 μm) match well the small physical size of tumor foci and micrometastases (10). α-Emitters have been considered for cancer therapy because their high-linear-energy transfer radiation is more effective at cell killing (11) than are the low-linear-energy transfer β-particles. When an α-emitter targets bone surfaces, α-particle crossfire into normal, healthy bone marrow from sources located on the bone surface should be substantially less than the crossfire from β-emitters that are currently approved for clinical use.
In a recent study with 211At and 131I linked to bone-seeking bisphosphonates, a high bone surface-to-bone marrow dose ratio was estimated for 211At relative to 131I (12). Other than 211At, only a few other α-particle-emitting radioisotopes are considered useful for biomedical applications (10). 212Bi has been evaluated as a bone-seeking agent (13,14). However, the short physical half-life of 212Bi (60 min) and relatively long times required for bismuth phosphonates to localize in bone result in higher normal-tissue exposure during the uptake and elimination phases (13,14). This effect is more pronounced with 213Bi (half-life, 46 min). The β-emitter 212Pb (half-life, 10.6 h) parent has been tried as an in vivo generator for 212Bi. However, a substantial redistribution of 212Pb and 212Bi was observed, resulting in high kidney accumulations of 212Bi (14). Other α-emitting radionuclides that are potentially useful in biomedical applications are 223Ra (half-life, 11.43 d) (15) and 224Ra (half-life, 3.64 d) (16).
224Ra was used medically for many years to treat ankylosing spondylitis (17–19). The bone-seeking properties of radium in humans are well known (17,18). However, the physical half-lives of 224Ra and its decay products are not ideal for targeting skeletal metastases. As demonstrated in animal experiments, a large fraction of the daughter products of 224Ra escape from bone (20,21) because of the noble gas daughter radionuclide, 220Rn (half-life, 55.6 s), which diffuses away from the site of 224Ra decay.
223Ra, another relatively short-lived radium isotope considered for cancer treatment, is likely more suitable as a bone-seeking radiopharmaceutical because its half-life (11.43 d) is about 3 times that of 224Ra. This longer half-life allows greater incorporation into bone surfaces before decay of 223Ra occurs. Thus, a smaller fraction of the total radiation dose will be delivered to soft tissues of the body during the uptake and elimination phase. In addition, the radon daughter, 219Rn, has a very short half-life (3.9 s), which means that the radon will not have sufficient opportunity to diffuse or migrate away from the site of 223Ra deposition.
During the decay of 223Ra and daughter radionuclides, 3 of the 4 α-particles are emitted almost instantaneously as 223Ra decays to stable 207Pb (Table 1). The last α-emitter in the 223Ra chain, 211Bi (half-life, 2.15 min) follows the decay of the β-emitter 211Pb (half-life, 36.1 min), and some biologic redistribution of the last 2 daughters may be expected. If 211Pb is trapped in the bone matrix, the α from 211Bi will irradiate the bone surface area.
α- and β-Radiation Emitted in Decay Chain of 223Ra
Experiments with 223Ra and 89Sr (half-life, 50.53 d) were conducted to determine relative uptake in soft tissues and bone. This article describes a dosimetric analysis of 223Ra and 89Sr on bone surfaces of mice and the degree to which surface deposits irradiate healthy red marrow.
MATERIALS AND METHODS
Radionuclides
223Ra was produced from an 227Ac generator system, as described elsewhere (22). Briefly, 227Ac and 227Th were immobilized on an actinide-selective resin to allow highly effective retention of actinium and thorium under conditions at which 223Ra elutes. The solution containing purified 223Ra was evaporated to dryness. The 223Ra activity was dissolved in isotonic saline with 5 mmol/L ammonium citrate, pH 7.4. Prior to injection, the solution was filtered through sterile 0.22-μm nylon filters (Nalgene, Rochester, NY).
γ-Radiation emitted by 223Ra and several of its decay products is useful for determining the quality and quantity of radionuclides in biologic samples. 223Ra has a characteristic γ-peak at 154.2 keV (5.59% abundance) (23), and 211Bi has a γ-peak at 351.1 keV (12.8%). These photons may be used to determine whether daughter products redistribute in living systems. 223Ra also has a 269.4-keV peak (13.6%), which is difficult to distinguish from the 219Rn peak at 271.23 keV (9.9%).
89Sr-Chloride (Metastron; Amersham Health, Buckinghamshire, U.K.) was diluted to the desired activity concentration with sterile saline solution.
Biodistribution Experiments
All procedures and experiments involving animals in this study were approved by the National Animal Research Authority (Norway) and were performed according to the European Convention for the Protection of Vertebrates Used for Scientific Purposes (24).
89Sr-Chloride and 223Ra-chloride were administered to mice by tail vein injection using 100–150 μL of solution. Young male BALB/c mice with body weights of 19–21 g were injected with 9 kBq of 223Ra or 3 kBq of 89Sr. Groups of mice were sacrificed and dissected at 1 h, 6 h, 24 h, 3 d, and 14 d after injection. Activity levels chosen were those that were deemed optimum for biodistribution analysis and counting on the analysis system at these time points. The tissue uptake of 223Ra and decay products was measured with the radionuclides in radioactive equilibrium; tissue samples were stored for at least 5 half-lives of 211Pb before the samples were measured. Two measurement procedures were used: In the first, tissue samples were counted using a NaI(Tl) well-type detector (Harshaw Chemie BV, De Meern, Holland) combined with a scaler timer ST7 digital unit (NE Technology Ltd., Reading, U.K.); in the second, samples were measured by liquid scintillation counting. In this procedure, soft-tissue samples were dissolved by adding 1–3 mL of Soluene 350 (Packard, BioScience BV, Groningen, Holland) per 100 mg of tissue. Bone samples were dissolved in HClO4:H2O2 1:2 (v/v). All tissue samples were maintained at 50°C until they were completely dissolved. If required, soft-tissue samples were bleached with H2O2. Insta-Gel Plus II scintillation cocktail (Packard) was added, and the samples were stored in the dark to avoid interference from room luminescence. The 89Sr content of mouse tissues was also determined by liquid scintillation counting, as described above for 223Ra.
Reference standards of 223Ra (in equilibrium with daughter radionuclides) and 89Sr were used during tissue analyses.
To investigate the redistribution of decay products from 223Ra, samples of blood, liver, and kidney were studied immediately after dissection using a solid-state germanium detector (Canberra, Meriden, CT) combined with an amplifier and bias supply from EG&G Ortec (Oak Ridge, TN).
Retention of Progeny from 223Ra Located in Bone
To examine the differences in radionuclide retention between 223Ra and 211Bi in bone samples, γ-spectroscopy was performed on bone samples and compared with data from a standard solution of 223Ra in equilibrium with its daughters. γ-Spectroscopy with the germanium detector was performed on samples of femur immediately after the mice had been sacrificed and dissected. The distinct γ-peaks at 351.0 keV (211Bi) and 154.2 keV (223Ra) were used for this analysis. For 223Ra, a localization index based on the relative counting rate (CR) of 223Ra and 211Bi in samples versus standard was determined as (CR of 211Bi in sample/CR of 211Bi in standard)/(CR of 223Ra in sample/CR of 223Ra in standard).
For statistical analysis, γ-spectra from 5 samples from the 6-h group and from the 3-d group, respectively, were compared with 5 and 3 samples from the standard solutions, respectively. The Student t test was used to determine statistical differences between the groups.
An additional experiment was conducted to investigate the potential release of 223Ra progeny from bone. Femurs from 5 mice sacrificed at 6 h and from 5 mice sacrificed 3 d after injection were examined. The bones were cleaved longitudinally, to expose the red marrow, and thereafter were cut into small pieces of less than 3 mg each. The bone samples were washed with Dulbecco’s phosphate-buffered saline (Sigma-Aldrich Co., Ltd., Irvine, U.K.) using centrifugation. The supernatant, including dissolved bone marrow, was removed, mixed with scintillation liquid (Insta-Gel Plus II), and counted on a scintillation counter (Beckman Instruments Inc., Fullerton, CA). Sample counting was repeated after 1 d. The difference in counts after correcting for 223Ra decay between the 2 measurements was used to identify the release of daughter radionuclides from bone matrix.
Dosimetry Calculations
Tissue absorbed doses for 223Ra (and daughters) and 89Sr were determined, and their localized energy distributions were calculated using both multicellular dosimetry and standard dose-averaging methods (25,26). The trabecular bone was modeled as a solid matrix of bone mineral containing a high density of spheric marrow cavities. The surface-to-volume ratios for bone, as described by the International Commission on Radiological Protection (27), were assumed to hold true for the mice in this study. Marrow cavities of 3 different spheric sizes (radii = 50, 150, and 250 μm) were considered, and doses were calculated assuming an activity of 0.67 Bq/mm2 223Ra and 20.42 Bq/mm2 89Sr on the bone surfaces of these cavities. The activities of 89Sr and 223Ra used in these calculations corresponded to those projected to result in a total absorbed dose of 10 Gy to bone. Instantaneous uptake and infinite biologic retention of the activity at bone surfaces were assumed.
Methods using the standard MIRD formalism were described previously by Fisher and Sgouros (28) for assessing the localized dosimetry of 223Ra and daughter products. Briefly, the absorbed dose is the product of all the energies emitted by 223Ra and daughters that are absorbed locally in the target mass, divided by the mass of the target, integrated over time. The principal α-, β-, and γ-ray emissions of 223Ra and daughters were included in this analysis. The absorbed dose (Gy) to a target region (rk) from a source region (rh) resulting from an internally administered radionuclide (29,30) is:
 Eq. 1 where Ãh is the cumulated activity or total number of transformations (Bq s) that have taken place in the source tissue (rh), and S (Gy Bq−1 s−1) is the mean dose to a target tissue (rk) per unit cumulated activity in the source tissue. The cumulated activity Ãh in a source region (rh) is the total number of radioactive transformations over time t (infinite time), accounting for both radioactive decay and biologic clearance:
Eq. 1 where Ãh is the cumulated activity or total number of transformations (Bq s) that have taken place in the source tissue (rh), and S (Gy Bq−1 s−1) is the mean dose to a target tissue (rk) per unit cumulated activity in the source tissue. The cumulated activity Ãh in a source region (rh) is the total number of radioactive transformations over time t (infinite time), accounting for both radioactive decay and biologic clearance:
 Eq. 2 The cumulated activity was determined by integrating a time-activity curve obtained from the counting results for each tissue using least squares regression.
Eq. 2 The cumulated activity was determined by integrating a time-activity curve obtained from the counting results for each tissue using least squares regression.
The S value is the product of the reciprocal mass mk (g) of the target organ or tissue and the sum of the products of the dose constant (mean energy emitter per nuclear transition, or Δi, in Gy kg Bq−1 s−1) and the energy absorbed fraction of the ith emission (φi):
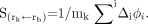 Eq. 3 The absorbed fraction (φi) is the fraction of energy emitted by the ith emission from activity in the source region that is absorbed in the target region. These fractions were calculated for the α- and β-energies characteristic of each of the major emissions from 223Ra and daughters, and from 89Sr. The equilibrium dose constant Δi is the sum of the products of a unit conversion constant (k), the fractional abundance of each emission (ni), and the energy of the ith emission (Ei):
Eq. 3 The absorbed fraction (φi) is the fraction of energy emitted by the ith emission from activity in the source region that is absorbed in the target region. These fractions were calculated for the α- and β-energies characteristic of each of the major emissions from 223Ra and daughters, and from 89Sr. The equilibrium dose constant Δi is the sum of the products of a unit conversion constant (k), the fractional abundance of each emission (ni), and the energy of the ith emission (Ei):
 Eq. 4 The equilibrium dose constant for 223Ra and daughters through complete decay of the 223Ra decay chain is 4.51 × 10−12 Gy kg Bq−1 s−1 (60.0 g rad μCi−1 h−1). A total of 28.2 MeV of energy is liberated by this decay chain, of which 93% is by α-particle emission.
Eq. 4 The equilibrium dose constant for 223Ra and daughters through complete decay of the 223Ra decay chain is 4.51 × 10−12 Gy kg Bq−1 s−1 (60.0 g rad μCi−1 h−1). A total of 28.2 MeV of energy is liberated by this decay chain, of which 93% is by α-particle emission.
The equilibrium dose constant for 89Sr is 9.32 × 10−14 Gy kg Bq−1 s−1 (1.24 g rad μCi−1 h−1). The mean energy of the β-particle from 89Sr is 0.583 MeV (Emax = 1.492 MeV). The absorbed fractions of β-energy in bone and soft tissues were determined using Berger’s point kernels for absorbed-dose distributions of β-particles in unit-density medium, scaled by interpolation to compact bone density (ρ = 1.85), and assuming point sources for 89Sr (31,32).
α-Particle doses with depth in marrow were calculated using the method described by Roeske et al. (25). Briefly, the dose D(r) at a particular point is given by:
 Eq. 5 where f(r→ − r→′) is the dose point kernel representing the dose deposited as a function of radial distance from a point source located at r→′. The quantity A(r→′) is the intensity of the source per unit area. The dose point kernel for 223Ra is given by:
Eq. 5 where f(r→ − r→′) is the dose point kernel representing the dose deposited as a function of radial distance from a point source located at r→′. The quantity A(r→′) is the intensity of the source per unit area. The dose point kernel for 223Ra is given by:
 Eq. 6 where ai is the fractional contribution from the ith α-particle, and dE/dx is the linear energy transfer function based on data from the International Commission on Radiation Units and Measurements (26). In using this form for the dose point kernel, we assumed that the α-particles travel in straight lines and lose energy through the continuous slowing-down approximation. Thus, negligible scattering and statistical fluctuations in energy deposition along the particle path were assumed valid. In addition, the range of δ-rays and the width of the α-particle track (∼100 nm) were ignored since the target that is often considered (i.e., cell nucleus) is much larger than these dimensions (33). Equation 5 was evaluated using numeric integration.
Eq. 6 where ai is the fractional contribution from the ith α-particle, and dE/dx is the linear energy transfer function based on data from the International Commission on Radiation Units and Measurements (26). In using this form for the dose point kernel, we assumed that the α-particles travel in straight lines and lose energy through the continuous slowing-down approximation. Thus, negligible scattering and statistical fluctuations in energy deposition along the particle path were assumed valid. In addition, the range of δ-rays and the width of the α-particle track (∼100 nm) were ignored since the target that is often considered (i.e., cell nucleus) is much larger than these dimensions (33). Equation 5 was evaluated using numeric integration.
RESULTS
Biodistribution of 89Sr and 223Ra
The uptake of 223Ra and 89Sr in selected tissues of mice is shown in Figures 1 and 2, respectively. Additionally, the uptakes of 89Sr, 223Ra, and decay products in samples of brain and heart were measured and found to be insignificant at all time points. The results showed that 223Ra selectively concentrated in bone, compared with the concentration in soft tissues. Although all the soft-tissue values decreased in radionuclide concentration between 1 h and 3 d after injection, the bone activity concentrations had already reached a level close to maximum after 1 h, with no statistically significant change in the levels during the 14 d. After 24 h, the percentage of injected dose per gram of femur tissue was 40.1 ± 7.7. For 89Sr, the corresponding value was 17.7 ± 2.8. At 14 d, the values for 223Ra and 89Sr were 31.1 ± 2.6 and 21.1 ± 2.7, respectively. For 223Ra, the femur-to-blood ratio increased from 118 to 691 within a period of 1 h to 3 d. Kidneys and spleen had the highest retention of 223Ra among the soft tissues. However, the femur-to-kidney ratio also increased with time, from 1.9 to 91 between 1 h and 3 d after injection. The femur-to-spleen ratio was also 1.9 at 1 h but increased to 24 at 3 d after injection. The femur-to-kidney and femur-to-spleen ratios of 223Ra were lower than the ratios for 89Sr.
Uptake of 223Ra in organs and tissues of mice. Columns represent mean ± SD of percentage injected activity per gram of tissue for groups of 5 mice per time point.
Uptake of 89Sr in organs and tissues of mice. Columns represent mean ± SD of percentage injected activity per gram of tissue for groups of 5 mice per time point.
Retention of Progeny from 223Ra Located in Bone
The average ratio of 211Bi to 223Ra in the spleen at 6 h was 0.54, compared with a standard solution. In liver and kidneys, the average 211Bi-to-223Ra ratios were, respectively, 2.56 and 2.07 times those of the standards. These results indicated that some migration of 223Ra daughters occurred in soft tissues. The 211Bi activity in soft tissues was low, compared with that in bone. The presence of 211Bi in soft tissues likely resulted from 223Ra in soft tissues.
In bone, the average localization index for the 211Bi-to-223Ra ratio was 0.85 (P = 0.059, n = 5) at 6 h and 0.97 (P = 0.749, n = 5) at 3 d; that is, the differences were not significant.
Mice sacrificed after 6 h showed some release of 223Ra daughter activity from bone. Compared with the total activity in bone, 1.8% dissolved (mean value) in phosphate-buffered saline during washing. When the washing solutions were counted again after 12 h, the activity was, on average, only 0.2% of that in the bone sample. This result indicated that less than 2% of the 223Ra daughter radionuclides migrated away from bone surfaces. Femurs from mice that were sacrificed after 3 d showed no significant 223Ra decay product counts, compared with background levels in the washing solution. This result indicated that migration of 223Ra decay products, if it occurred, was less than our detection limit and also less than about 1% of the total radioactivity in bone.
Dosimetry
As described above, the radionuclide source cumulated activities were calculated by integrating the time-activity curves obtained from the 223Ra and 89Sr biodistribution studies. The animal biodistribution data (Fig. 1) confirmed that the assumption of instantaneous uptake and infinite retention in bone was a valid approximation since both radionuclides reached an activity level equivalent to maximum only 1 h after injection and the radioactivity levels in bone did not change significantly up to 14 d. Table 2 shows our absorbed dose estimates to bone and soft-tissue samples (mGy) per 1 kBq administered per gram of body weight. As can be seen from Table 2, dosimetry indicated a greater radiation absorbed dose to soft tissues per kilobecquerel of activity for 223Ra than for 89Sr because of the lower decay energy of 89Sr than for 223Ra.
Radiation Absorbed Dose Estimates
The mean of the radiation absorbed doses to femur, skull, and rib (Table 2) was used as an average dose estimate for bone. Based on this value, the activity needed to produce an absorbed dose to bone of 10 Gy was calculated. From the activity value and from the absorbed dose estimates (mGy/kBq per gram of body weight; Table 2), the corresponding absorbed doses to soft tissue were calculated. Table 3 shows the absorbed doses to soft tissues for a total estimated absorbed dose to bone of 10 Gy. This method predicts higher radiation doses to the kidneys, lungs, intestines, and spleen from 223Ra than from 89Sr.
Absorbed Doses Estimated for Soft-Tissue Organs
Figure 3 shows the results of small-scale, localized dose calculations for 223Ra and 89Sr distributed on the bone surface of marrow cavity spheres having radii of 50, 150, and 250 μm. The activities of 89Sr and 223Ra used in these calculations corresponded to those projected to result in a total absorbed dose of 10 Gy to bone. For 223Ra, the estimated absorbed dose in a 250-μm sphere decreased steeply from approximately 65 Gy at 5 μm from the surface to 0 Gy at about 70 μm, the energy-range cutoff distance. For a 150-μm sphere, the absorbed dose decreased steeply with distance from 75 Gy at 3 μm to 0 Gy at 69 μm. For a 50-μm sphere, the absorbed dose decreased from 97 Gy near the bone surface to about 60 Gy in the least exposed volume.
Dose estimates for 223Ra and 89Sr distributed on surface of spheres of radii 50, 150, and 250 μm. Activity of 0.67 Bq/mm2 was assumed for 223Ra, and activity of 20.42 Bq/mm2 was assumed for 89Sr.
By comparison, only small changes in absorbed dose from 89Sr, with distance from surface, were observed from the dose calculations. The implications of this dosimetry are important to understanding the potential differences between 223Ra and 89Sr with respect to marrow toxicity. This work shows that the principal advantage of α-particle emitters for targeting osteoid surfaces is that substantial volumes in the trabecular marrow cavities would receive very low radiation doses from 223Ra, compared with 89Sr, per unit bone surface dose and that the sparing of normal red marrow would have important clinical significance for the patient. 223Ra could produce a clinically more effective and substantially greater bone surface dose for therapeutic benefit while minimizing the radiation absorbed dose and associated hematopoietic suppression associated with localized irradiation of red marrow.
DISCUSSION
A desirable approach to treating metastatic cancer in the skeleton with bone-seeking radionuclides would be to use radiopharmaceuticals that deliver a high dose to the bone lesions while sparing bone marrow. Because of their short range corresponding to the physical dimensions of single cancer cells and micrometastases, high-linear-energy transfer, dose-rate independence, and effective cell-killing efficiency (34), α-emitters have outstanding potential for treating metastatic cancer. Compared with other α-emitting radionuclides for targeted radiation therapy, 223Ra has several favorable properties. 223Ra may be produced relatively inexpensively and in large amounts from 227Ac (half-life, 21.7 y), which can be obtained by neutron irradiation of 226Ra target material. The relatively long physical half-life of 223Ra (11.4 d) is advantageous for generating source material, shipping material, and preparing and administering the radiopharmaceutical yet is short enough to avoid generating long-lived radioactive hospital waste. The use of 223Ra in clinical settings is also favored from a practical standpoint. Compared with therapy with other radionuclides, 223Ra requires less shielding because the fraction of penetrating radiation is low for the 223Ra decay series. Radiation exposure to medical staff would therefore be much less for 223Ra than for several other α-emitting radionuclides.
In skeleton-targeted therapy, the rapid α-particle cascade from 223Ra has potential to deliver high radiation doses to tumors. With regard to concerns on the redistribution of daughter products, any potential application of 223Ra must account for the potential increased radiation exposure to normal organs and tissues. Thus, the biologic distribution of both the parent radionuclide and the decay products must be considered carefully. In the present study, bone samples from mice injected with 223Ra were studied by γ-ray spectroscopy, and the relative bone content of 211Bi versus 223Ra was determined immediately after dissection. The results of this research indicated that only very small amounts of 223Ra daughter radionuclides redistributed from the site of 223Ra decay in bone. Based on the extractable radioactive fraction from finely fragmented bone samples, it was found that the outward translocation of 223Ra daughter products from the bone matrix was low. Short-lived activity was observed in the supernatant from finely fragmented bone samples at 6 h. However, the released activity at 3 d after injection was estimated to be less than 1% of the total bone content of 211Pb and 211Bi. This finding indicated that even for the 211Bi transformation (the fifth transformation in the series from 223Ra), the retention of decay products in bone was similar to that of the parent 223Ra. Additionally, this finding represents an important argument to counter the concerns over redistribution of 223Ra daughter products from the targeted tissue. Prior studies of 224Ra in beagles showed a significant redistribution of 212Pb and 212Bi from 224Ra-deposited bone (20). The retention of 212Pb and 212Bi in bone increased with time and reached a plateau at approximately 80% and 70% of their equilibrium values, respectively, at about 2 d after injection (20). The present study on 223Ra and the existing data on 224Ra produced the following conclusions about the differences between 223Ra and 224Ra when used as bone-targeting agents: First, 223Ra has a longer physical half-life than 224Ra, which shifts the bone-to-soft-tissue ratios to higher values because a larger fraction of 223Ra is eliminated from soft tissue before decay occurs. Second, the longer half-life of 223Ra than of 224Ra allows greater incorporation into the bone surfaces during bone remodeling. This effect may contribute to better retention of daughter products, which could otherwise redistribute because of diffusion and α-recoil. Third, the radon daughter of 223Ra (219Rn) has a shorter half-life than that of 224Ra (220Rn), resulting in substantially less redistribution of daughter radionuclides from the 223Ra series.
In the current study, bone uptake of 223Ra in mice was found to be high and selective compared with uptake in the soft tissues. In addition, 223Ra was well retained in bone. The biologic elimination of 223Ra from soft tissues was rapid, compared with its half-life. 223Ra was found to have a high bone uptake, confirming work by others that showed radium to be an excellent bone-seeking element. This study used normal mice. Alternatively, tumor-bearing animals could have been used. The current study represents an initial effort to evaluate 223Ra as a potential treatment for bone metastases. Because few data have been published on 223Ra, we chose to use normal immune-competent animals without tumors to study the basic metabolism of the compound, since animals with skeletal metastases could have significantly altered biokinetics due to increased bone metabolism in the tumor area. Also, 223Ra could be particularly effective to sterilize the bone surfaces against microdeposits of cancer cells (e.g., in the preosteoblastic stage of metastasis development). Several studies have focused on the use of agents to prevent development of future skeletal events (35–37). It is therefore interesting to evaluate, as a prelude to future experiments in tumor models, whether the normal bone uptake of 223Ra could deliver substantial doses with the potential to sterilize bone-surface-deposited micrometastases (38).
Doses were estimated using macroscopic and small-scale dosimetric techniques. The results of dose calculations from the biologic data showed that for a given dose to bone, 223Ra provided a greater dose to some soft tissues (kidneys, spleen) than did 89Sr (Table 2). However, 223Ra also provides a high, intensely localized radiation dose at bone surfaces (Fig. 3), meaning that a relatively low administered activity will produce a therapeutically relevant dose with little consequence to kidneys and spleen. Moreover, the concentration of radiation energy close to bone surfaces results in substantial sparing of healthy red marrow, the critical organ for normal-tissue toxicity in skeleton-targeted radionuclide therapy of osteoid neoplasms. With assumed marrow cavity sizes of 150 and 250 μm in trabecular bone, and assuming 0.67 Bq/mm2 of 223Ra, an estimated dose to the surface of about 70 Gy is delivered. On the basis of these estimates, healthy red marrow in the adjacent bone cavities will receive total radiation doses that are much lower than those resulting from 89Sr under comparable circumstances, allowing a greater fraction of marrow cells to survive treatment. That relatively large amounts of the 223Ra can be tolerated has been indicated in a pilot study in which mice injected with 1 kBq of 223Ra per gram of body weight were followed for 3 mo without showing signs of life-threatening acute toxicity (38). This finding indicates that bone marrow and soft-tissue organs could withstand therapeutically relevant levels of 223Ra.
In the current study, we used a simplistic geometric representation of the bone marrow cavities to compare the relative merits of 2 radionuclides. A more sophisticated analysis would involve performing 3-dimensional dosimetry calculations based on realistic tumor and bone marrow geometries. Such analyses for bone marrow have been performed by Akabani and Zalutsky (39) and by Charlton et al. (40). In both cases, histologic samples of bone marrow were obtained from either beagles (39) or cadavers (40). Histologic bone marrow and tumor samples would provide additional insight beyond this study, such as the maximum tumor size that could be treated with 223Ra. However, in terms of comparing the relative merits of these radionuclides, we would expect results similar to those obtained in this report.
CONCLUSION
223Ra may provide both a more effective radiation source and a safer radiation source for red marrow than does 89Sr. Dose estimates in this study supplied additional justification for 223Ra as a novel approach to treating micrometastatic cancer deposited on the surfaces of the skeleton. Compared with the current suite of β-emitters used in palliation of bone pain, 223Ra localized at bone surfaces (and perhaps taken up by calcified, osteogenic tumors) can, together with its daughter radionuclides, deliver an intense and highly local radiation dose of α-particles with bone marrow sparing. Further studies—for example, on an appropriate skeletal metastasis model—are therefore warranted.
Acknowledgments
The authors thank Solveig Garmann Vik and Marita Martinsen at the Norwegian Radium Hospital for their skillful assistance with the animal experiments.
Footnotes
Received Sep. 28, 2001; revision accepted Mar. 25, 2002.
For correspondence or reprints contact: Roy H. Larsen, PhD, Anticancer Therapeutic Inventions AS, P.O. Box 54, Kjelsaas, N-0411 Oslo, Norway.
E-mail: roy.larsen@ati-as.no









