Abstract
Gallbladder ejection fraction (GBEF) measured with a fatty meal (half-and-half milk) was compared with that measured with 2 equal sequential intravenous infusions of cholecystokinin (CCK-8) in a paired study of healthy subjects. Methods: GBEF was measured by 99mTc-hepatic iminodiacetic acid cholescintigraphy in 13 healthy subjects. Each subject received 2 sequential doses of CCK-8 (3 ng/kg/min for 10 min) on day 1, followed by, on day 2, a 240-mL (8 oz) fatty meal (half-and-half milk) per 70 kg of body weight. Results: The mean ± SD GBEF of 53.6% ± 20.2% with fatty meal was significantly lower than the mean of 75.8% ± 16.3% (P < 0.01) with the first dose of CCK-8 and 71.3% ± 17.4% (P < 0.05) with the second dose. Fatty meal GBEF varied widely, from 23.5% to 91.8%. Percentile rankings of the fatty meal GBEF were determined as the preferred methodology for reporting results. Latent and ejection periods were significantly longer with fatty meal than with either dose of CCK-8. Conclusion: GBEF measured with fatty meal can serve as an alternative method to intravenous injection of CCK-8 when the hormone is no longer available for clinical use. The measurement of GBEF with fatty meal requires careful attention to the details of the meal and the measurement time sequence.
Quantification of function is an essential part of hepatobiliary imaging in general and biliary dyskinesia in particular (1). Measurement of gallbladder ejection fraction (GBEF) with intravenous infusion of exogenous cholecystokinin (CCK) is a well-established technique for the diagnosis of biliary dyskinesia, which includes both chronic acalculous cholecystitis (CAC) and sphincter of Oddi spasm (SOS). Biliary dyskinesia is characterized primarily by functional alterations without accompaniment of morphologic changes (2). A low GBEF is the characteristic feature of CAC. Reflux into the intrahepatic ducts of bile emptied from the gallbladder during CCK infusion, and subsequent paradoxic filling of the gallbladder immediately after cessation of octapeptide of CCK (CCK-8) infusion are the main features of SOS. These are well-established parameters using an intravenous infusion of CCK-8 as the stimulus. A total of 30 min of data collection with CCK-8 is usually sufficient to generate all the necessary parameters required for diagnosis (3). A fatty meal, which releases endogenous CCK, also has been used as a stimulant to empty the gallbladder (4,5). A direct comparison between CCK-8 and fatty meal, however, has not been made in a sufficient number of healthy individuals to contrast and compare these 2 stimuli. With the current shortage and impending nonavailability of CCK-8 (sincalide for injection, or Kinevac; Bracco Diagnostics, Inc., Princeton, NJ), such a comparison has become more of a necessity than ever before (6). This project was undertaken to assess in a paired study the effects of dose and duration of infusion of CCK-8 or of fatty meal contents on the magnitude of gallbladder emptying in healthy subjects.
MATERIALS AND METHODS
The study was performed at the Veterans Administration Medical Center (Portland, OR) in 1989 and 1990. Participants were recruited through an advertisement placed in a university newspaper soliciting paid participants in a research study conducted to obtain gallbladder data in healthy subjects. Chosen subjects denied having any abdominal pain, had normal liver function, had normal liver and gallbladder on ultrasound examination (no gallstones), and were not taking medications that would affect gallbladder emptying (e.g., opioids). Each subject was studied twice (Fig. 1), once with 2 equal sequential doses of CCK-8 and, on a separate occasion, with a half-and-half milk fatty meal (HHFM). The purpose of the study was explained, and informed consent was obtained on a form approved by the hospital committee for human studies. Women in the reproductive age group were studied within 10 d after the onset of the last menstrual period. Because hormone replacement therapy (HRT) was controversial at the time, postmenopausal women on HRT were excluded.
Protocol used with CCK-8 and fatty meal study. Gallbladder was filled to full volume with 99mTc-HIDA during first 60 min of hepatic-phase imaging. Two doses of CCK-8 were infused sequentially for 10 min, beginning at 65 (CCK1) and 95 min (CCK2). Fatty meal was ingested in sitting position just before beginning gallbladder-phase data collection at 61 min in supine position.
After 6–8 h of fasting, each subject underwent cholescintigraphy, receiving 111 MBq (3 mCi) 99mTc-hepatic iminodiacetic acid (HIDA) intravenously while lying supine underneath a large-field-of-view gamma camera fitted with a low-energy, all-purpose, parallel-hole collimator. Hepatic-phase images were obtained at 1 frame per minute for 60 min. Gallbladder-phase image data were collected separately at 1 frame per minute for another 60 min (61–120 min). Both studies were recorded on a 64 × 64 × 16 computer matrix. Two equal doses of CCK-8 (denoted as CCK1 and CCK2) were administered sequentially. Each CCK-8 dose consisted of 3 ng/kg/min administered intravenously for 10 min through an infusion pump. Infusion of the first dose (CCK1) was started at 65 min after 99mTc-HIDA injection and the second dose (CCK2) at 95 min after 99mTc-HIDA injection. The study with the fatty meal was performed on another day. The dose of half-and-half milk used as the HHFM was adjusted for body weight (240 mL [8 oz]/70 kg of body weight) and ingested in the sitting position, just before gallbladder-phase data collection in the supine position (Fig. 1). Each HHFM included 24 g fat, 8 g carbohydrate, 8 g protein, and 320 calories.
A count-based GBEF was obtained as described previously with CCK-8 and fatty meal (3,4). The latent period (time from beginning of CCK-8 infusion or HHFM ingestion to beginning of gallbladder emptying), ejection period (EP, time from beginning to the end of gallbladder emptying), and ejection rate (ER, percentage ejection fraction [EF] divided by EP) were noted for each subject. Because the gallbladder was still emptying at 60 min after the meal in most subjects, GBEF was calculated at 60 min (Fig. 2). Differences in EF, EP, and ER with CCK1, CCK2, and HHFM were subjected to repeated-measures ANOVA with subsequent Newman-Keuls analysis of pairs. P < 0.05 was considered statistically significant (7).
Types of gallbladder emptying curves seen with fatty meal. Most common type of curve is represented by x; y curve shows no latent period because of cephalic phase (nervous control or sham feeding) of gallbladder emptying; x shows long latent period; and z shows short latent period. Curves w, x, and y show gallbladder in emptying phase when data collection was terminated at 60 min after meal.
RESULTS
A total of 21 subjects entered the study. We defined a GBEF of 35% as the lower limit of normal with a 3-min infusion of 3.3 ng/kg/min CCK-8. Study participants were required to have a GBEF of at least 35% with CCK-8 to be included as truly healthy subjects. This requirement would exclude subjects with possible asymptomatic gallbladder disease (e.g., cholesterolosis). Seven men and 1 woman with GBEF < 35% on CCK-8 were excluded from analysis, leaving 13 individuals (8 women, 5 men; age range, 24–53 y) who fulfilled all of the preset criteria for healthy subjects. Table 1 and Figures 3–5 provide the details of the statistical analysis for EF, EP, and ER.
GBEF obtained with CCK1, CCK2, and HHFM. Note that only 1 subject had GBEF < 60% with CCK1 and only 1 had < 50% with CCK2. GBEF values vary more with fatty meal.
Gallbladder Ejection Fraction in 13 Healthy Subjects*
Repeated-measures ANOVA (which tests whether the gallbladder function parameter under consideration is dependent on the gallbladder stimulus method) showed a statistically significant difference between CCK-8 (both CCK1 and CCK2) and fatty meal in EF, EP, and ER (null hypothesis: CCK1 = CCK2 = HHFM; P = 0.006 for EF; P < 0.001 for EP and ER). The subsequent Newman-Keuls statistical analysis then further tests for differences between pairs of gallbladder stimulus methods (i.e., null hypothesis: CCK1 = CCK2; null hypothesis: CCK1 = HHFM; or null hypothesis: CCK2 = HHFM).
GBEF is constant for a fixed dose of CCK-8. Our data indicate that the gallbladder empties to the same extent each time when given 2 equal doses of CCK-8 (CCK1 and CCK2). The latent period was <2 min with both CCK1 and CCK2. Mean SDs of GBEF with CCK1 and CCK2 were 75.8% ± 16.3% and 71.3% ± 17.3%, respectively, and these EF values were not significantly different from each other (P > 0.05; Fig. 3). The EPs of 13.8 ± 4.4 min with CCK1 and 13.6 ± 6.4 min with CCK2 also were not significantly different from each other (Fig. 4). The ERs (%EF/min) with CCK1 and CCK2 were 6.1% ± 2.5%/min and 6.1% ± 2.6%/min, respectively, and these rates were not significantly different from each other (Fig. 5).
Gallbladder EP seen with CCK1, CCK2, and HHFM. Note wider variability with HHFM than with CCK1 or CCK2.
Gallbladder ER (%GBEF/min) obtained with CCK1, CCK2, and fatty meal. Note that fatty meal produces lowest rate of emptying.
GBEF is variable with HHFM. HHFM produces lower and more variable EF than does a 10-min infusion of CCK-8. The fatty meal mean EF at 60 min of 53.6% ± 20.2% was significantly lower than the mean value obtained with either CCK1 (P < 0.01; EF = 75.8% ± 16.3%) or CCK2 (P < 0.05; EF = 71.3% ± 17.3%). EF variability was much greater with HHFM than with either CCK1 or CCK2 (Fig. 3). The latent period with fatty meal ranged from 10 to 40 min. The fatty meal EP of 34.6 ± 11.0 min was significantly longer (P < 0.01) than the EP with CCK1 or CCK2 (Fig. 4). The fatty meal ER of 1.8% ± 1.1%/min was significantly lower (P < 0.01) than the ER with either CCK1 or CCK2 (Fig. 5).
DISCUSSION
These results indicate that an intravenous infusion of CCK-8 induces gallbladder emptying almost immediately and that a constant dose given sequentially 2 or 4 times (8) with 30-min intervals between doses produces similar degrees of gallbladder emptying. The GBEF is dependent on the dose, dose rate, and duration of infusion of CCK-8 and can be controlled to any desired level simply by varying the duration of infusion of the hormone. Within the physiologic range, the relationship between CCK-8 dose and GBEF is linear, and the EF begins to fall when this dose limit is exceeded. Infusion over 3 min of 1, 5, and 10 ng/kg CCK-8, for example, produces mean GBEFs of 0%, 36%, and 50%, respectively. A dose of 10 ng/kg CCK-8 infused over 3 min (3.3 ng/kg/min) shows the peak emptying effect without eliciting any untoward effects in healthy subjects (9). Infusion of a higher dose, such as 20 or 40 ng/kg, reduces mean EFs to 29% or 36%, respectively. The intravenous CCK-8 dose recommended in the sincalide package insert (20 or 40 ng/kg) was developed in the early 1970s using oral cholecystogram and is too high for cholescintigraphy. About 26% of healthy subjects given 20–40 ng/kg CCK-8 over 3 min develop abdominal pain, and many show no gallbladder emptying at all (9). A high CCK dose is known to induce contraction of the cystic duct with subsequent nonemptying of the gallbladder (10).
An identical dose of CCK-8 administered twice, sequentially, produces similar degrees of gallbladder emptying, an observation that has been reported previously (11). Because of its short serum half-life of only 2.5 min, the effect of exogenous CCK-8 on the gallbladder wears off completely 15–20 min after cessation of infusion. Neither a facilitating nor an inhibitory effect from the previous dose is manifested on the subsequent CCK-8 dose, when at least 30 min is allowed between doses. Sequential dose study results indicate that the gallbladder empties as long as serum CCK-8 level is maintained above the threshold for contraction and stops emptying soon after the infusion ceases and the serum level falls below the threshold within 15–20 min. The gallbladder resumes emptying immediately (within 2 min) after a second CCK-8 infusion is begun. These types of results may enable the study of the effect of various drugs or interventions on the gallbladder or sphincter of Oddi. The EF of the first CCK-8 dose serves as the basal value, and response to the identical second dose after the drug or intervention indicates the effect of therapy.
The mean GBEF values obtained with CCK1 and CCK2 are higher than the mean obtained with HHFM at 60 min after the meal (Fig. 3). Unlike CCK-8, with a latent period < 2 min, the gallbladder latent period with HHFM is much longer and ranges from 6 to 26 min (4). The longer latent period is probably the result of time taken for release of endogenous CCK. Endocrine cells that secrete CCK are distributed primarily in the mucosa of the duodenum, jejunum, and proximal ileum. No CCK-secreting endocrine cells are found in the mucosa of the oropharynx, esophagus, stomach, or beyond the distal ileum and colon (12). Ingested food, therefore, must reach the duodenum before it is able to release endogenous CCK. Fatty meal stimulation creates some problems that need attention during quantitative cholescintigraphy. Because of the longer latent period and EP with HHFM, the duration of data collection should be for a minimum of 60 min after the meal. This longer duration of data collection reduces the throughput of the gamma camera. Delay in gastric emptying could affect the results in some patients, and lactose intolerance may become a concern in some children. These problems, however, are relatively rare in adults, in whom biliary dyskinesia is more common. Greater variability in latent period, EP, and EF with HHFM than with CCK1 or CCK2 may be related to variation in both time and quantity of endogenous CCK released into circulation.
A 10-min intravenous infusion of CCK-8 produces significantly more bile emptying than does 240 mL (8 oz) HHFM per 70 kg of body weight at 60 min. Unlike the action of the left ventricle, which has a constant resting EF at any given time, GBEF is controllable to any desired level simply by altering the dose, dose rate, or duration of infusion of CCK-8 or by altering total dose, calories, protein, or fat content in the meal. Serum endogenous CCK levels remain elevated above the threshold for gallbladder contraction for 90–180 min after the meal (13). GBEF obtained with the fatty meal, therefore, depends not only on the meal contents but also on the duration of postmeal data collection. EF has been measured at 30, 60, 90, or 120 min after the meal, with varying GBEF values (1,5). With data collection at 60 min after the meal, the GBEF curve usually shows a downward slope, suggesting that emptying is not complete when data collection is terminated (Fig. 2). Therefore, it is essential not only to adjust fatty meal dose for body weight but also to report GBEF value with a time reference, such as GBEF at the end of 30, 60, 90, or 120 min after the meal.
The type of fatty meal available locally may have some effect, thereby causing regional variation in GBEF. Comparison of nutrient content in half-and-half milk from 6 cities in the United States is shown in Table 2. Because the nutrient content of half-and-half milk is similar in many of the brands, it appears that GBEF values developed in one region of the country may be applicable in another, as long as the dose is adjusted for body weight. Gallbladder emptying also can be studied with sequential exogenous and endogenous CCK stimulation after a single dose of 99mTc-HIDA (14).
Nutrient Content and Total Calories of Half-and-Half Milk in 6 U.S. Cities
Many different methods are used to establish normal ranges and identify individuals needing treatment. In studies involving a large sample of patients in whom the frequency distribution of population data resembles a gaussian curve, it is traditional to use mean ± 2 SDs or 95% confidence limits to set the upper and lower limits of the normal range. Data in our 13 healthy subjects using HHFM showed a wide GBEF range, from 23.5% to 91.8%. The 95% confidence interval using mean ± 2 SD would show GBEF values from 13% to 94%. Such a wide range of normal GBEF values is unlikely to separate healthy individuals from patients with gallbladder disease. Use of mean ± 2 SDs as a confidence range is valid only for data that follow a gaussian distribution. The number of subjects in our study is too small to demonstrate whether GBEF with CCK-8 or fatty meal follows a gaussian distribution in healthy subjects. Such problems are relatively common in clinical medicine, where the results for hormone or drug levels in the blood often show a large amount of overlap between healthy subjects and patients. When the data do not follow a gaussian distribution, alternative methodology sometimes is used to define the normal range. An arbitrary cutoff level may be chosen and tested to see how this affects false-positive and false-negative results in a mixed sample of healthy subjects and patients. The cutoff value can be adjusted to any desired level. Clinical judgment is then used to define the cutoff value that determines the best mix of false-positive and false-negative results. We cannot use this methodology now, because our current data include only healthy subjects.
We suggest using percentile rank methodology to convey the clinical import of a GBEF measured with fatty meal. This type of approach is used in measurement of bone mineral densitometry, in which practitioners have come to use not so much the absolute numeric value of the bone density but, instead, a percentile rank for each patient compared with healthy subjects. Figure 6 shows the percentile rank versus the GBEF for HHFM and CCK-8. The percentile rank for HHFM is calculated from the regression equation:
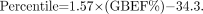 For example, a patient with a measured HHFM GBEF of 40% would be reported in the 28th percentile compared with healthy subjects (Fig. 6A). Instead of an absolute cutoff, the percentile rank of the patient’s GBEF is reported, and the clinician then uses other clinical information to assess the importance of GBEF in designing therapy. An alternative approach would be to set a GBEF lower limit at 30% and accept a slightly lower sensitivity for the test.
For example, a patient with a measured HHFM GBEF of 40% would be reported in the 28th percentile compared with healthy subjects (Fig. 6A). Instead of an absolute cutoff, the percentile rank of the patient’s GBEF is reported, and the clinician then uses other clinical information to assess the importance of GBEF in designing therapy. An alternative approach would be to set a GBEF lower limit at 30% and accept a slightly lower sensitivity for the test.
Percentile rank for HHFM-induced GBEF (A) and for CCK-8–induced GBEF (B). Note much smaller variation in GBEF with CCK-8 than with HHFM.
The serum level of exogenous CCK-8 declines rapidly within 15–20 min after cessation of its infusion and allows rapid refilling of the gallbladder in patients with SOS. Recognition of gallbladder paradoxic filling with CCK-8 enables noninvasive diagnosis of SOS (2). This type of response suggests that one must acquire data for at least 15–20 min after cessation of CCK-8 infusion to be able to see paradoxic filling associated with SOS. At this time, the imaging characteristics of the paradoxic filling of the gallbladder in SOS patients after a fatty meal are not known.
Currently, the only product approved by the U.S. Food and Drug Administration for clinical use is Kinevac. Most nuclear medicine practitioners have by now received a letter from the manufacturer announcing the impending shortage and nonavailability of CCK-8, possibly for a year or more. We, as well as others, have received many phone calls from colleagues asking how to cope with this shortage. This is an unfortunate turn of events, occurring at a time when our clinicians are fully convinced of the usefulness of quantitative hepatobiliary imaging and are requesting more frequent 99mTc-HIDA studies for their patients. We believe that HHFM is an acceptable alternative to CCK-8. It is promising to note that many compounding pharmacies have come forward to supply sincalide when the request is made by a physician on a patient prescription form (6). This is certainly a timely and welcome advance.
CONCLUSION
A 10-min intravenous infusion of 3 ng/kg/min CCK-8 produces a higher GBEF than does 240 mL (8 oz) HHFM per 70 kg of body weight at 60 min after the meal. The results of a GBEF measurement with fatty meal should be reported in terms of the percentile rank compared with healthy subjects. The percentile rankings refer specifically to 240 mL (8 oz) half-and-half milk per 70 kg of body weight as the fatty meal stimulus for GBEF at 60 min after ingestion. HHFM stimulation can serve as an alternative when CCK-8 is no longer available for clinical use.
Acknowledgments
This project was partially supported by the Veterans Affairs Department and a grant from Squibb Diagnostics, Princeton, NJ. The authors thank Ramegowda Rajagopal, MD (Detroit, MI), Vishwanath Hebbar, PhD (Miami, FL), Jamuna Murthy, MS (Los Angeles, CA), and Kalpana Krishnamurthy, BA (New York, NY), for providing information related to half-and-half milk content from their cities and Mr. Bob Crummett, graphic designer, Tuality Healthcare, Hillsboro, OR, for preparing the figures.
Footnotes
Received Apr. 8, 2002; revision accepted Jul. 26, 2002.
For correspondence or reprints contact: Gerbail T. Krishnamurthy, MD, Tuality Community Hospital, 335 SE 8th Ave., Hillsboro, OR 97123.
E-mail: GTKrishna@aol.com












