Visual Abstract
Abstract
Cardiac PET imaging is increasingly used for myocardial perfusion studies because of its high diagnostic accuracy and low radiation exposure to the patient. However, patient motion can be challenging, affecting a large number of studies. Motion artifacts can lead to inconclusive or false-positive results, complicating clinical interpretation. This article explores the causes of motion artifacts and their characteristic appearance in cardiac PET imaging, highlighting their distinction from true perfusion abnormalities. Strategies for minimizing motion through effective patient positioning and communication are discussed. Understanding and addressing motion artifacts are crucial for optimizing diagnostic accuracy and ensuring the full benefit of cardiac PET imaging.
Cardiac PET myocardial perfusion imaging use has increased throughout the United States over the past 10 y (1). This expansion is attributed to its multiple advantages, including higher diagnostic accuracy, faster scan protocols, lower patient and staff radiation exposure, and fewer artifacts, resulting in fewer false-positive studies (2). Unfortunately, cardiac PET is prone to motion arising from the heart, breathing, cardiac creep, and patient movement, potentially producing inconclusive results (3). This article will examine preventable patient motion during PET imaging with 82Rb, the characteristic appearance of motion artifacts, and the consequential impact on clinical interpretation.
PATIENT MOTION CAUSE AND PREVENTION
Even though 82Rb acquisitions are substantially faster than a 99mTc SPECT, requiring only 7 min, patients still move during the rest or stress acquisition, causing image degradation. Body movement or gross patient motion occurs because of physical discomfort such as arm discomfort due to hyperextension, discomfort associated with the pharmacologic stress agent administration, anxiety, talking, coughing, deep breathing, settling, or gradual relaxation of thoracic muscles (4).
The reported occurrence of patient body motion ranges from 30% to 69% of cardiac PET studies, particularly during the stress acquisition (4). Preventing patient motion requires both thorough preacquisition instructions by the nuclear medicine technologist and consistent reminders during the acquisition. Clear communication about the importance of remaining still significantly improves patient compliance throughout the data acquisition.
CARDIAC SPECT VERSUS PET MOTION ARTIFACT
Although motion artifact occurs with both SPECT and PET, motion artifact presents distinct challenges in cardiac PET. First, continuous data acquisition in PET imaging precludes frame-by-frame motion correction capabilities that are available with SPECT. Consequently, motion artifacts manifest more frequently in processed PET images. Furthermore, the absence of rotating projection images in PET eliminates a valuable tool for motion identification available in SPECT imaging. Thus, motion during PET acquisition cannot be detected until after image reconstruction.
Historically, cardiac PET and PET/CT imaging have lacked robust solutions for patient motion correction despite motion’s well-documented impact on diagnostic accuracy and quantitative measurements. However, technologic advances have led several equipment manufacturers to develop and implement motion correction algorithms for newer-generation scanners (5,6).
RECOGNIZING PET MOTION ARTIFACT
When examining cardiac PET imaging, motion artifacts typically present with distinctive characteristics—appearing irregular, patchy, and sometimes with a characteristic “slitlike” appearance. Although these artifacts generally affect the entire study, they can occasionally manifest more localized, with the apical segments particularly susceptible. It is worth noting that mild to moderate motion typically does not mimic the appearance of coronary artery disease.
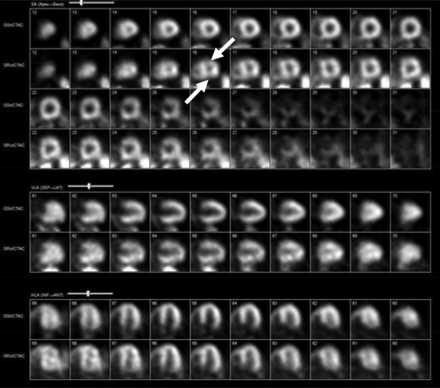
Motion can occur during either the rest or the stress acquisition or on both acquisitions. Figure 1 provides an excellent illustration of rest-phase motion. In this case, the stress portion demonstrates good quality with a normal perfusion pattern and no defects. The rest portion, however, demonstrates generalized motion. Looking at the apical slices, particularly the short-axis views, contralateral defects affect both the anterior and inferior walls. The defects are small and discrete, with characteristics differentiating them from true perfusion abnormalities associated with coronary artery disease.
Mild motion artifact. Motion artifact on rest images with normal perfusion at stress. Rest images demonstrate irregular perfusion with contralateral defects in anterior and inferior walls (arrows). Contralateral defects do not conform to typical coronary artery disease distribution patterns.
Further, the left ventricular cavity appears elongated, representing another characteristic sign frequently associated with patient movement. Given that the stress portion demonstrates normal perfusion, the study can be confidently interpreted as entirely normal despite the motion artifact observed in the rest images.
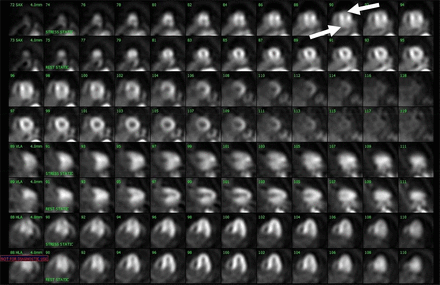
Motion artifacts in cardiac PET imaging vary and are sometimes quite severe. When severe, these artifacts affect the entire ventricle from the apex to the base and distort the left ventricle shape. They often manifest as contralateral defects in the anterior and inferior walls, with 1 defect (usually the inferior) appearing more pronounced than the other. These findings make interpretation challenging, if not impossible, and may necessitate repeat imaging (Fig. 2).
Severe motion artifact. Stress images demonstrate severe motion artifacts affecting slices from apex to base, most prominent in apical and inferior walls, with distorted left ventricular shape. Study with this degree of motion should be repeated.
CT ATTENUATION CORRECTION (TRANSMISSION)/EMISSION MISALIGNMENT
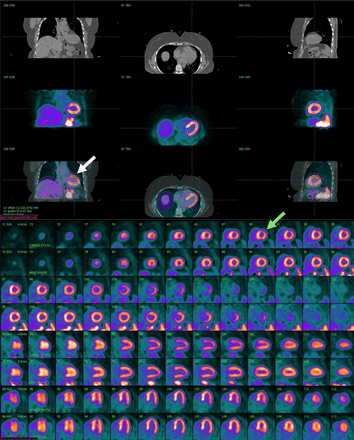
Another concern for motion artifacts is the misalignment of the CT attenuation correction (transmission) and PET emission images (7). Because the attenuation correction and emission scans are acquired sequentially, patient motion on the emission imaging can cause attenuation correction artifacts. The extent and direction of the misalignment will determine whether artifacts will be apparent on the attenuation-corrected images (Fig. 3). Most commercial systems have software tools to correct transmission–emission misalignments.
CT attenuation correction/emission misalignment. Mild misalignment of stress CT transmission and 82Rb emission images (white arrow) resulted in anterior and anterolateral walls overlapping lung field (green arrow) on stress images. Consequently, stress–rest myocardial perfusion images show small anterior and anterolateral reversible defect (arrow).
EFFECT OF MOTION ON MYOCARDIAL BLOOD FLOW QUANTIFICATION
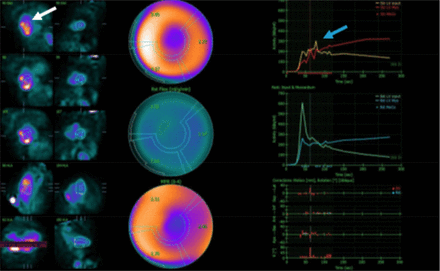
Patient motion can substantially affect myocardial blood flow quantification (8). For accurate interpretation, the dynamic sequence (blood flow acquisition) must be free of patient motion to ensure the blood pool region of interest remains properly centered within the left atrial or left ventricular cavity throughout the entire acquisition (Fig. 4). When reviewing time–activity curves, multiple peaks suggest patient movement during critical study phases.
Myocardial blood flow quantification motion. Severe patient motion during stress dynamic acquisition shows incorrect position of region (white arrow) and multiple peaks (blue arrow) on time–activity curves, making data inaccurate and uninterpretable.
Before proceeding with myocardial blood flow calculation, any motion should be addressed through manual or automated software correction algorithms. This quality control step is essential for maintaining diagnostic accuracy for quantitative perfusion measurement.
INTERPRETATION STRATEGIES FOR STUDIES WITH MOTION ARTIFACT
When patient motion is encountered in 82Rb cardiac PET studies, a systematic approach to interpretation is essential. Generally, once a motion artifact has been identified and the perfusion pattern clearly does not conform to a typical coronary artery distribution, the study can confidently be interpreted as normal (3).
Whether to mention motion artifacts in the final report is at the discretion of the interpreting physician and depends on their confidence level in distinguishing artifacts from pathology. In rare cases, a motion artifact may be so severe that it precludes the definitive exclusion of coronary artery disease. In such cases, the interpreting physician must decide whether to classify the study as equivocal or recommend a repeat study with technologist guidance to minimize motion.
CONCLUSION
Despite its relatively short acquisition time, patient motion remains a significant challenge in cardiac PET imaging with 82Rb. Motion occurs in 30%–69% of studies, particularly during the stress acquisition. Nuclear medicine technologists are crucial in minimizing this artifact through effective patient preparation and communication.
The unique characteristics of PET imaging—continuous data acquisition and lack of rotating projection images—make motion artifacts more challenging to identify and correct compared with SPECT. However, recognizing the distinctive appearance of motion artifacts allows technologists to confidently differentiate them from true perfusion abnormalities.
Although newer-generation scanners increasingly incorporate motion correction algorithms, prevention remains the optimal approach. When motion is detected, careful evaluation of image quality is essential to determine whether the study can be interpreted as normal or must be repeated.
Nuclear medicine technologists can prevent motion artifacts and optimize image quality and diagnostic accuracy by understanding the causes, appearance, and implications of patient motion in cardiac PET imaging. This ultimately ensures that patients receive the full benefit of this advanced imaging modality.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: By evaluating the causes, characteristic appearances, and strategies for prevention and interpretation, can limiting patient motion on cardiac PET imaging enhance diagnostic accuracy?
PERTINENT FINDINGS: Patient motion in cardiac PET imaging can significantly impact diagnostic accuracy, with motion artifacts affecting many studies. Effective patient positioning and communication are crucial in minimizing motion.
IMPLICATIONS FOR PATIENT CARE: Patient motion during cardiac PET imaging can lead to inconclusive or false-positive results, potentially delaying diagnosis and treatment, emphasizing the critical role of nuclear medicine technologists in preventing these artifacts through effective patient positioning and communication.
Footnotes
Published online Apr. 22, 2025.
REFERENCES
- Received for publication March 14, 2025.
- Accepted for publication March 25, 2025.