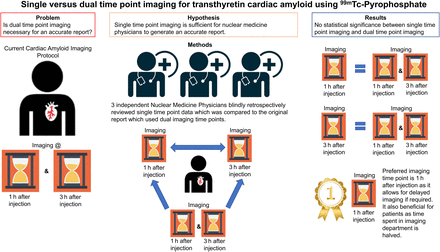
Visual Abstract
Abstract
Nuclear medicine scintigraphy using 99mTc-pyrophosphate has proven valuable in the diagnosis of cardiac transthyretin amyloidosis in recent years. However, there is still confusion over the optimal imaging time points. The American Society of Nuclear Cardiology has recommended different imaging time points over the last decade. We aimed to determine whether single- or dual-time-point imaging is required for reporting purposes and which time point would be the most appropriate if a single time point was to be considered. Methods: Cardiac amyloid scans using 99mTc-pyrophosphate acquired from 2017 to 2023 were retrieved from our Picture Archiving and Communications System. Scans with static views and SPECT/CT images of the chest for both imaging time points, at 1 h (early) and 3 h (delayed) after injection, were included. Each study was independently read by 3 nuclear medicine physicians. Original clinical reports using both imaging time points were used as a reference to calculate the accuracy of a single time point. Results: In total, 70 patients were included in this study. Reports of cardiac amyloid studies using any single-time-point imaging were highly sensitive, accurate, and specific. There was agreement among all readers. Of the 140 datasets reported by each reader, 4 scans were classified as equivocal, requiring more imaging for confident reporting. Conclusion: Single-time-point imaging showed an accuracy comparable to the dual-time-point imaging in diagnosing cardiac transthyretin amyloidosis. This was further validated by agreement among the 3 readers. Early time-point imaging is preferred, and additional delayed imaging can be acquired when the early result is equivocal.
Amyloidosis is a systemic condition which can affect several organs, including the kidneys, brain, liver, and heart, and can be fatal if untreated (1). There are 2 main types of cardiac amyloidosis, transthyretin amyloidosis (ATTR) and light chain amyloidosis. The diagnosis of cardiac amyloidosis can be challenging because of the wide range of symptoms that are similar to those of other conditions and the requirement of several tests to confirm the presence of amyloidosis (2). This study focuses on cardiac ATTR. The gold standard for the diagnosis of cardiac amyloidosis is endomyocardial biopsy (3). The invasive nature of this procedure, which requires highly skilled medical professionals, can lead to severe complications such as bleeding, arrhythmias, infection, perforation of the heart, and damage to heart valves or blood vessels (4). An array of diagnostic tools is being used where possible to accurately diagnose cardiac amyloidosis without endomyocardial biopsies. These tools include but are not limited to cardiac MRI, echocardiography, electrocardiography, serum biomarkers, and nuclear medicine scintigraphy (5–7). 99mTc-pyrophosphate (99mTc-PYP) scintigraphy has proven valuable in the diagnosis of cardiac ATTR in recent years with high sensitivity and specificity ranging between 97%–100% and 93.3%–100%, respectively (8–10).
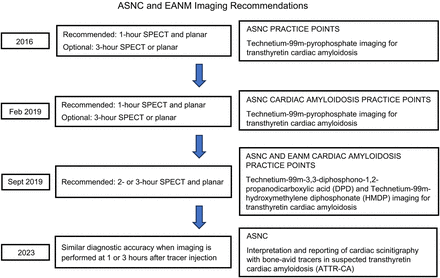
The timing of the nuclear medicine scans after the administration of 99mTc-PYP is not a standardized practice across nuclear medicine departments. The uptake mechanism of pyrophosphate in ATTR is not very well understood, but it is believed that it is related to the presence of elevated calcium levels in amyloid-deposited tissues (11). The American Society of Nuclear Cardiology (ASNC) originally released a 99mTc-PYP imaging practice points in 2016, with an updated version in February 2019 (Fig. 1). The 2019 version recommends images to be acquired at 1 h after injection, and if more information is needed, additional images to be acquired 3 h after injection (12). In September 2019, an ASNC and European Association of Nuclear Medicine Cardiac Amyloidosis DPD (diphosphono-1,2-propanodicarboxylic acid) Practice Points adapted from the February 2019 version was released. This adaptation focused on the different radiopharmaceuticals that are more widely used in Europe, 99mTc-DPD and 99mTc-hydroxymethylene diphosphonate. The recommended imaging time points were 2 or 3 h after injection, with both planar and SPECT imaging and the 1-h time point being optional (13). Most recently, in 2023, ASNC released an interpretation and reporting document that found similar diagnostic accuracy for both imaging time points of 1 and 3 h. SPECT/CT was found to be essential for the accurate reporting of cardiac amyloidosis regardless of the time point, whereas semiquantitative analysis is optional (14).
Timeline of recommendation from ASNC and EANM.
We aimed to determine if single- or dual-time-point imaging is required for reporting purposes and which time point would be the most appropriate if a single time point was to be considered.
MATERIALS AND METHODS
The current protocol in our department is to image the patient at 2 time points, at 1 and 3 h after injection, acquiring planar images and at least 1 SPECT/CT image (Table 1). A definitive diagnosis of cardiac amyloidosis is often established by endomyocardial biopsy; however, this is not always performed. For our study, a diagnosis of cardiac ATTR was established by reviewing the clinical history, diagnostic tests, results from patients’ electronic medical records, and original clinical reports of the PYP scans, which used both imaging time points. Most patients had diagnostic tests including echocardiogram, blood tests, cardiac MRI, and a PYP scan to investigate the presence of cardiac ATTR. Ethics approval for this retrospective study was granted by Austin Health Human Research Ethics Committee, and the requirement for obtaining informed consent was waived.
There were 207 consecutive patients who had 99mTc-PYP cardiac amyloid studies performed on a GE Discovery 670DR (GE HealthCare) and reviewed. The 99mTc-PYP scans were retrieved from the hospital’s AGFA Picture Archiving and Communication System. Only patients who had static chest planar and SPECT/CT imaging of the chest at both imaging time points were included in this review. In total, 70 patients met these criteria and were thus included. Each study was split into 2 separate datasets, early and delayed datasets. All the datasets were anonymized, and time stamps were erased, processed, and reviewed by 3 readers, 2 qualified nuclear medicine physicians and 1 nuclear medicine trainee, masked to the patients’ clinical history and imaging time points.
Acquisition Parameters for 99mTc-PYP Scans
A semiquantitative analysis was performed on the static chest planar image. Two regions of interest of the same size were drawn over the heart and the contralateral right lung (Fig. 2). A ratio was calculated by dividing the counts from the heart regions of interest by the counts from the contralateral lung regions of interest. The ratio was noted on the saved image for the specialist to review. At our center, as per the 2019 ASNC practice points, a ratio of 1.5 or more on the early static image and uptake within the myocardium on SPECT/CT was considered a positive study (12). The ASNC PYP Practice Points (12) does not stipulate a cutoff ratio value for delayed imaging, but Scully et al. (15) suggest a cutoff value of 1.3, with a ratio of 1.3 or more indicative of a positive study for the delayed time point.
Example of positive study, with ratio of heart region of interest (ROI) vs. contralateral lung region of interest being more than 1.5.
All image processing was done by a single experienced nuclear medicine technologist. The processed data were independently reviewed by each reader who reported the datasets as positive, negative, or equivocal. A case was recorded as positive if there was a mention of diagnosis or treatment of cardiac ATTR in the patient’s medical records. No hospital medical records could be found for 2 patients, who had external follow-up. Excluding these 2 cases, the data were used to calculate the specificity of a single time point of 99mTc-PYP cardiac amyloid scans.
Statistics
The percentage agreement among all 3 readers and the 2 experienced readers was calculated. The interobserver variability was corrected for chance between any 2 readers and assessed using Cohen κ coefficient at the 95% CI. The interobserver variability among all 3 readers was evaluated using the weighted Fleiss κ coefficient (16). A κ value of less than 0.01 would be considered no agreement, 0.01 to 0.2 poor, 0.21 to 0.40 fair, 0.41 to 0.60 moderate, 0.61 to 0.80 substantial agreement, and 0.81 to 1.00 good agreement.
Statistical analysis of the datasets was performed using the Statistical Package for the Social Sciences (IBM Corp.).
RESULTS
Population Cohort
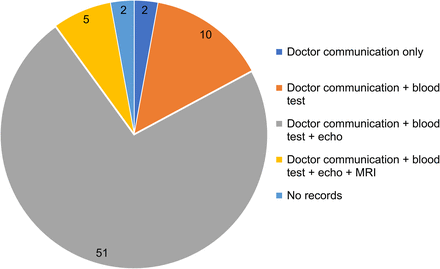
Twelve patients had confirmed diagnosis of cardiac amyloidosis. The diagnosis of 10 patients was confirmed via doctor communication, blood tests, and echocardiograms; 1 patient’s diagnosis was confirmed via doctor communication, blood tests, echocardiograms, and cardiac MR, and 1 patient’s diagnosis was confirmed via doctor communication and blood test only (Fig. 3). Demographics and indications for cardiac amyloid scans for patients who had a SPECT/CT chest scan at both time points are shown in Table 2.
Source of clinical data for diagnosis of possible cardiac amyloidosis.
Characteristics of Patients in Cohort
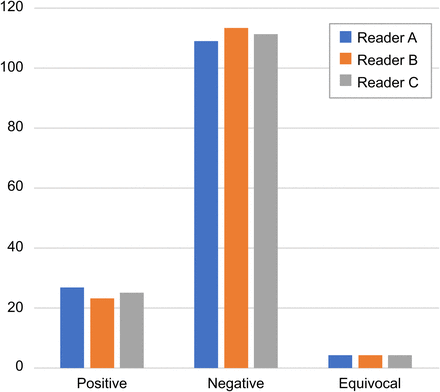
All 3 readers had 4 equivocal datasets from the 140 datasets (70 for the early imaging time point and 70 for the delayed imaging time point). The equivocal datasets were not necessarily the same for all readers (Fig. 4). The sensitivity of reporting using the early imaging point was 100% for all 3 readers. The sensitivity of reporting using the delayed dataset only was 100%, 90.9%, and 100% for reader A, reader B, and reader C, respectively. The accuracy of reporting using the early dataset only was 95.7%, 97.1%, and 95.7% for reader A, reader B, and reader C, respectively. The accuracy of the report using the delayed dataset only was 95.7%, 94.2%, and 97.1% for reader A, reader B, and reader C, respectively. The specificity of the reporting using the early dataset was 96.4%, 98.2%, and 98.2% for reader A, reader B, and reader C, respectively. The specificity of reporting using the delayed dataset was 98.2% for reader A and reader B and 98.3% for reader C (Table 3). The agreement among all 3 readers was 95.7% and 92.8% for early dataset and delayed dataset, respectively. The agreement between the 2 senior readers, reader A and reader C, was 95.7% for both datasets. The Fleiss κ at 95% CI for all 3 readers was 0.91 for the early time point and 0.83 for the delayed time point, which is categorized as good agreement. The Cohen κ at 95% CI for the 2 senior readers was 0.88 and 0.87 for the early and delayed time points, respectively, which is again categorized as good agreement.
Scans results reported by each reader (readers A and C were senior physicians).
Sensitivity, Accuracy, and Specificity of Using Single Dataset for Reporting 99mTc-PYP Cardiac Amyloidosis
The difference in sensitivity, accuracy, and specificity between early and delayed imaging was not statistically significant, with P values of 0.422, 0.741, and 0.400, respectively.
DISCUSSION
One of the challenges of reporting 99mTc-PYP cardiac amyloid scans is the accumulation of the radiopharmaceutical in the chambers of the heart on early images, and it is challenging to distinguish between myocardial uptake and blood pooling on static planar images only. For cases in which blood pooling is seen and suspected on early images, delayed images can be acquired, as blood pooling is expected to be cleared by 3 h, or an early SPECT/CT can be acquired to distinguish between myocardial uptake and activity in the heart chambers (11). There appears to be varying approaches regarding optimal imaging time in the current literature. Many nuclear medicine departments are using the 3 h time point for reporting (9,10,17–19), but some recent studies have demonstrated that scans can be reported at the 1-h time point with equal confidence (8,20).
The results of our study show that the difference in sensitivity and specificity of reporting of any single imaging time point is not statistically significant when compared with using both imaging time points for reporting (P > 0.05). Furthermore, the difference in accuracy of reporting early images only versus delayed images only is not statistically significant (P > 0.05). The results show that experienced readers have 100% sensitivity for both time points. The junior reader (trainee) had 100% sensitivity for the early dataset compared with 90.9% for the delayed dataset. This drop in sensitivity was due to a single false-negative case in the delayed dataset. The experience levels of readers in nuclear medicine and the strong interobserver agreement demonstrate that both imaging time points are not necessary for all patients. Our study is in agreement with the work done by Bokhari et al. (8) who investigated several factors affecting image quality, including matrix size, counts per image, and imaging time points and chose the 1-h time point because of its excellent image quality and lower extracardiac activity. It also supports the findings of Masri et al. (20) who demonstrated that little additional information was gained when images were acquired at both time points. The diagnostic accuracy for each time point is equally high.
Castano et al. (21) and Schatka et al. (22) both investigated the sensitivity of cardiac amyloid scans in nuclear medicine, using the gold standard endomyocardial biopsy as reference and demonstrated higher sensitivity at the 1-h time point. 99mTc-PYP clears from the bloodstream quickly, and it is taken up by the bone and myocardium (23). The uptake of 99mTc-PYP in the myocardium reaches its peak around the 1-h mark, which could be a contributing factor to the higher sensitivity mentioned above (24).
The median age onset is commonly after the age of 40 but not usually diagnosed until a geriatric age (25). At our center, it was observed that the patient population tested for cardiac amyloidosis is primarily elderly (26), many of whom require assistance with daily activities and are typically accompanied by a family member. In this cohort of patients, tolerance for long and tedious diagnostic procedures is low. The time between a patient arriving at the department and completing the early images is approximately 2 h. If the delayed images are acquired, at a minimum, this time is doubled. Because of the decrease in mobility of patients in this age group, they are usually not willing to leave the department between the early and delayed images, and an area needs to be found to accommodate them in nuclear medicine departments. Space limitations are not uncommon in many departments. Therefore, single-time-point imaging is more tolerable for the patients and preferred in our imaging department.
It is important to note that, in some cases, delayed imaging does play a significant role and can provide additional information and increase the confidence level of the reporting physician. In our review, 2 scans were classified as equivocal on the original reports. These 2 scans were reexamined by an independent senior nuclear medicine physician (not part of the original reader team), who deemed one of the equivocal readings as negative and confirmed the other as equivocal. This example demonstrates the reporting challenges in a few cases even when both early and delayed images were available. In our study, 9 patients (12 datasets) had a dataset reported as equivocal among the 3 readers. This result is very encouraging as it suggests that single-time-point imaging is adequate for most cardiac amyloid studies and only in a few difficult cases will need 2 imaging time points.
Limitations
Endomyocardial biopsy is the gold standard for the diagnosis of cardiac amyloidosis (27). Because of the invasive nature of this procedure, it is not commonly performed, and none of the patients included in this study had an endomyocardial biopsy. For this reason, information obtained from the patients’ medical records was used to confirm their cardiac ATTR status which was then used to calculate the specificity for single-time-point imaging. This investigation was a single-center, retrospective study.
CONCLUSION
The findings of our study demonstrate that single-time-point imaging, at either 1 or 3 h after injection, is sufficient for reporting most cardiac amyloid studies, which aligns with the recommendations of the most recent ASNC reporting and interpretation guideline (13) and the findings of recent literature (8,19). Early time-point imaging is sufficient for most cases, as supported by the high sensitivity, specificity, and diagnostic accuracy of our study, with the option of delayed imaging in equivocal cases. SPECT/CT acquisition at the early imaging time point plays a critical role in the reporting of cardiac amyloid scans and can help avoid the need for dual-time-point imaging.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Are 2 imaging time points necessary for cardiac ATTR imaging, and if so, which is the preferred time point?
PERTINENT FINDINGS: This retrospective analysis demonstrates that the difference in sensitivity, specificity, and accuracy between single- and dual-time-point imaging is not statistically significant. The early and delayed imaging time points also revealed similar sensitivity, specificity, and accuracy.
IMPLICATIONS FOR PATIENT CARE: Most patients referred for cardiac amyloid imaging are elderly and require a family member or a caregiver to accompany them to their appointments. The length of the imaging study has more than halved with single-time-point imaging at the 1 h mark.
Footnotes
Published online Apr. 22, 2025.
REFERENCES
- Received for publication January 5, 2025.
- Accepted for publication March 20, 2025.