Abstract
Objective: This article presents a brief synopsis of the 1997 regulations from the Nuclear Regulatory Commission concerning the release from the hospital of patients treated for thyroid disease with 131I. A simplified checklist is provided to demonstrate the instructions to patients and the new conditions for release of patients containing a higher level of 131I radioactivity than was allowed under the older regulations.
The Nuclear Regulatory Commission (NRC) revised Title 10 of the Code of Federal Regulations (10 CFR 35.75) in 1997 to allow the release of patients on a solely dose-based basis (1). This change permits a patient to be released from the hospital provided the total effective dose equivalent to any individual (other than the treated patient) will not exceed 500 mrem. Furthermore, in a case where the dose could exceed 100 mrem, the patient also is to be provided with instructions on how to maintain doses to others as low as reasonably achievable (ALARA). This rule was adopted to remove the inconsistency that existed with the promulgation of a 100-mrem public dose limit in Part 20 in 1991, with the previous administered radioactivity-based limit in the pre-1977 10 CFR 35.75. The guidance for implementation was published as NRC Regulatory Guide 8.39 (2). Note that this applies only to NRC licensees, however. Persons working in agreement states must check with their particular state regulators to ascertain whether their state has adopted the NRC's new guidelines.
This article provides the reader with a simple worksheet for releasing patients treated with Na131I. We first summarize the key points presented in NRC Regulatory Guide 8.39 and then provide some sample worksheets. We consider only patients administered Na131I either for treatment of hyperthyroidism or post-thyroidectomy for thyroid cancer. For other cases and for the extra considerations required for patients who are nursing an infant or child, please refer to the Regulatory Guide (2).
As shown in Table 1 (which is a synopsis of Table 4 from the Regulatory Guide), there are 4 bases for the release of patients: administered activity, retained activity, measured dose rate, and patient-specific dose calculations.
The first, second, and fourth bases in Table 1 apply an equation from NCRP Report No. 37 (3) to calculate the exposure to persons from the released patient. Using this equation, with the 131I physical half-life (8 d), an occupancy factor of 0.25 at 1 m, and assuming no shielding, an administered activity of ≤ 33 mCi Na131I will result in an exposure of ≤ 500 mrem. However, using the same model, the revised NRC regulations now require that instructions on “actions to maintain doses to other individuals as low as reasonably achievable” be given to patients released with greater than 7 mCi. This is because the exposure to any other individual from the released patient could exceed the ALARA limit of 100 mrem for administered activity of 131I as small as 7 mCi. Thus (basis 1 in Table 1), one may always release a patient administered 33 mCi or less of Na131I; however, instructions must be provided to the patient if he or she gives more than 7 mCi.
We will not discuss release bases 1–3 any further, because these are very similar to the older regulations (pre-1997) and probably very similar to the state regulations that apply for licensees in agreement states that have not as yet adopted the new NRC regulations. We presume that the major interest of the reader is to easily calculate the considerably higher levels of 131I (i.e., > 33 mCi) that now permit release of the patient under the patient-specific dosimetry (basis 4 in Table 1), so we now review the NRC guidelines for patient-specific dose calculations and, lastly, provide some simplified checklists.
For administered activities of Na131I greater than 33 mCi, patient-specific calculations must be performed and documented to allow the release of the patient. Patients may be released, regardless of the administered mCi of 131I, if these patient-specific calculations show a dose to any person, other than the patient, of <500 mrem. Incorporated into each patient-specific calculation must be considerations of occupancy factors, effective half-lives, and uptake fractions (based on a 2-component, extrathyroidal and intrathyroidal model of 131I pharmacokinetics). The formula to be used for the calculation of maximum likely dose to any individual exposed to the released patient (equation B-5 in the Regulatory Guide) is:
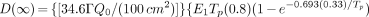
 where:
where:
D(∞) = dose to any person exposed to the patient (rem);
Γ = the exposure rate constant for 131I = 2.2 (R/mCi-hr at 1 cm);
Qo = the administered activity of Na131I (mCi);
E1 and E2 = the occupancy factors for the extrathyroidal and intrathyroidal components, respectively (see below);
F1 and F2 = the uptake fractions for the extrathyroidal and intrathyroidal components, respectively (see below);
TP = physical half-life for 131I = 8.04 d;
T1eff and T2eff = effective half-lives (in days) for the extrathyroidal and intrathyroidal components, respectively (see below).
The uptake fractions and effective half-lives can be measured experimentally for each patient, or alternatively taken from Table 2. The basis for them is explained in Regulatory Guide 8.39. Note that if you have an experimental measurement that supports a different value than that shown in Table 2, then you can use that in the equation for calculating D(∞).
The occupancy factor E is the fraction of time that any other person is considered to be located within 1 m of the patient. What can be used for the occupancy factor E? Regulatory Guide 8.39 suggests the following:
Use E = 0.75 when a physical half-life, an effective half-life, or a specific time period under consideration (e.g., bladder holding time) is ≤1 d
or
Use E = 0.25 when an effective half-life is greater than 1 d if the patient has been given instructions, such as the following:
Maintain a prudent distance from others for at least the first 2 days;
Sleep alone in a room for at least the first night;
Do not travel by airplane or mass transportation for at least the first day;
No travel on a prolonged auto trip with others for at least the first 2 d;
Have sole use of a bathroom for at least the first 2 d; and
Drink plenty of fluids for at least the first 2 d.
or
Use E = 0.125 when an effective half-life is greater than 1 d if the patient has been given instructions such as the following:
The instructions 1–6 for E = 0.25 above;
Live alone for at least the first 2 d; and
Have few visits by family or friends for at least the first 2 d.
Appendix A is a sample worksheet that can be used to document patient release, basically using the mCi administered to the patient along with the other numeric values as suggested in the Regulatory Guide to calculate the dose to any other person, D(∞). If this dose is <500 mrem, the patient may be released (along with appropriate patient instructions and record keeping requirements). Appendix B is a sample form that can be used to document patient and family instructions.
There are 2 final comments. First, there is some useful discussion of patient release that may be reviewed on the Health Physics Society web site (4). Second, there was a recent journal article (5) that supports the NRC's position that release of patients, using certain criteria, does not exceed the intended exposure limits.
Using the assumptions as stated in the Regulatory Guide, patients who are treated with ≤ 56 mCi 131I for hyperthyroidism, or patients treated with ≤ 220 mCi 131I for post-thyroidectomy treatment of cancer may be released from hospital confinement. All you need to do is document your instructions and calculations. Appendices 1 and 2 are examples of forms that can be used for documentation.
Summary of Release Criteria, Required Instructions to Patients, and Records to Be Maintained*
Uptake Fractions and Effective Half-Lives for Iodine-131*
Radioiodine Therapy Outpatient Worksheet
Instructions to Patients Containing More Than 6.9 mCi Na131I Released from the Medical Center
Footnotes
For correspondence or reprints contact: William K. Tuttle, III, PhD, Radiation Safety Office, P-5-FMS-RSO, Portland VA Medical Center, 3710 SW US Veterans Hospital Rd., PO Box 1034, Portland, OR 97207; Phone 503-220-8252, X55853; E-mail: william.tuttle{at}med.va.gov.







