Abstract
Gastric emptying scintigraphy (GES) as now commonly performed measures only total gastric emptying. Intragastric meal distribution (IMD) immediately after meal ingestion (t = 0 min) (IMD0) can assess fundic accommodation, and dynamic antral contraction scintigraphy (DACS) can assess antral motility. Our goals were to incorporate IMD and DACS into GES, compare IMD0 using gastric division into anatomic proximal and distal halves versus more physiologic separation of the antrum from the proximal stomach using DACS, and establish reference values. Methods: Healthy subjects (n = 20) underwent GES using a solid–liquid meal. DACS (1 frame/3 s) was performed for 20 min after each static imaging time. IMD0 was measured using both semiautomated software to divide the gastric long axis into anatomic halves and Fourier analysis to identify antral pixels with phasic contractions. Results: Using halving of the stomach, IMD0 averaged 0.75 ± 0.15 (SD). Using phasic contractions to define the antrum, mean IMD0 was 0.85 ± 0.14 (P = 0.004). Sustained antral contractions started at a mean of 11.24 ± 12.98 min after meal ingestion and originated in the gastric midbody with a starting location at 40.5% ± 10.8% from the distal to the proximal stomach along its long axis. Antral frequency and ejection fraction peaked 30 min after meal ingestion at 3.30 ± 0.71 contractions per minute and an ejection fraction of 30.3% ± 13.69%, when mean antral filling peaked at 36.7% ± 14%. Maximum antral contraction speed was 3.54 ± 0.90 mm/s at 60 min after meal ingestion. Gastric retention was 39.8% ± 12.8% at 2 h and 5.8% ± 6.0% at 4 h. Conclusion: Addition of DACS to GES permits physiologic characterization of both fundic accommodation and antral contractility to supplement routine GES.
See an invited perspective on this article on page 137.
Gastric emptying scintigraphy (GES) as currently performed is used to assess total gastric emptying. In standard GES, anterior and posterior static images are taken every hour after meal ingestion over 4 h (1). Processing of the static images requires decay correction, geometric mean correction of the total gastric counts in the anterior and posterior images to compensate for depth correction, and then normalization of total gastric counts to 100% immediately after ingestion (t = 0 min) to calculate the percentage of gastric counts remaining in the entire stomach at each time point (2). GES as now typically performed, however, for many symptomatic patients results in normal total gastric emptying (3). This problem has resulted in increasing interest in obtaining more information on the role of impaired fundic accommodation and antral dysmotility to explain symptoms and help direct targeted therapy (4).
Measurement of total gastric emptying does not adequately reflect the separate functions of the proximal and distal stomach. The fundus, functionally the proximal portion of the stomach, normally undergoes receptive relaxation and accommodation and then generates sustained pressure to move solids into the antrum. In response, the antrum then performs rhythmic contractions to break down the solid food particles to permit emptying of the solids through the pylorus.
Proximal gastric function and fundic accommodation can be assessed with routine GES imaging. Intragastric meal distribution (IMD) is defined as a measurement of how much food is in the proximal stomach compared with the food in the whole stomach. To use IMD as a measure of fundic accommodation, the IMD immediately after meal ingestion, or IMD0, has previously been calculated by anatomic division of the stomach into halves (5,6) or thirds (7).
Measurement of antral contractions can also be obtained as a part of GES with the addition of continuous dynamic imaging. Dynamic antral contraction scintigraphy (DACS) has been previously described and uses continuous imaging typically at 1 image per 3 s (8–10). Using an antral region of interest (ROI) and Fourier analysis, this can yield information on the frequency of antral contractions and assessment of the strength of antral contractions by measuring an antral ejection fraction. Although not previously described, an additional advantage of DACS is its ability to use the phasic and rhythmic contractions of the antrum to functionally separate the antrum from the noncontractile proximal stomach and thus permit a more physiologic measurement of IMD0 than is possible with simple anatomic division of the stomach.
The ability to measure abnormal fundic accommodation and abnormal antral motility using GES is important because they may potentially explain dyspeptic symptoms that are not explained by GES as currently performed (11,12). Studies using the gastric barostat suggested that impaired fundic accommodation is associated with early satiety and weight loss (13). Although the barostat study is considered the gold standard for assessing fundic accommodation, it is invasive and not widely available. In addition, the barostat balloon itself can alter gastric physiology (14). Several alternate methods have been developed to measure fundic accommodation, such as SPECT and MRI, but these are not in widespread use or use technology not widely available (14).
The primary aim of this study was to develop an acquisition protocol and software for GES that would permit measurement of IMD and DACS frequency and amplitude measurements in routine, clinical GES. Secondary aims were to establish reference values for IMD and DACS and to compare measurement of IMD0 using DACS to physiologically define the antrum versus simple anatomic division of the stomach.
MATERIALS AND METHODS
After obtaining institutional review board approval for this study of healthy volunteers, we recruited subjects to undergo GES with DACS. These subjects gave written informed consent and were questioned to ensure they had no prior history of gastrointestinal disease or dysfunction, had no prior gastrointestinal surgery, and were not taking medications that might affect gastrointestinal function.
GES
Subjects came to the nuclear medicine department in the morning after fasting overnight. GES was measured using the 4-h, liquid-egg-white protocol described initially by Tougas et al. (15) and currently recommended by the consensus report of the Society of Nuclear Medicine and Molecular Imaging and the American Neurogastroenterology and Motility Society (1). The meal consists of 120 g (4 oz.) of liquid egg white radiolabeled with 74.0 MBq (2.0 mCi) of 99mTc-sulfur colloid served with 2 pieces of white bread and jelly. In addition, patients were given 120 mL of water immediately after ingestion of the solid portion of the meal. The current Society of Nuclear Medicine and Molecular Imaging procedure guideline for GES (version 3.0) recommends up to a 37-MBq (1.0 mCi) dose of 99mTc-sulfur colloid (2). The higher dose used for this study was to ensure adequate count rates for analysis of the DACS based on prior experience (16).
After meal ingestion, static imaging using a 128 × 128 matrix was performed at 0, 0.5, 1, 2, 3, and 4 h, with the subject upright in the anterior and then the posterior position each for 30 s. Subjects then underwent DACS imaging as previously described (16). This consisted of continuous anterior imaging (1 frame/3 s) for 20 min beginning immediately after each of the anterior and posterior static image sets. For the DACS imaging, each subject was allowed to choose whether an upright standing or seated position was most comfortable and then instructed to remain as still as possible in that position for the 20 min of DACS imaging. In between the imaging times, the subjects sat in the nuclear medicine waiting area.
Before processing of the DACS images, the continuous dynamic images were reviewed and motion-corrected to eliminate any motion artifacts using GE Xeleris (GE Healthcare) motion correction software.
All GES images were analyzed using image processing software developed for this project. The software was developed using Matlab (MathWorks). A more detailed and technical description of the software is provided in the supplemental appendix (supplemental materials are available at http://jnmt.snmjournals.org). The overall image processing included measurement of the following:
Total Gastric Emptying
Static image processing included standard decay correction, geometric mean attenuation correction, and calculation of the percentage remaining at each time point (2).
IMD Based on Partitioning of Proximal and Distal Stomach by Halves
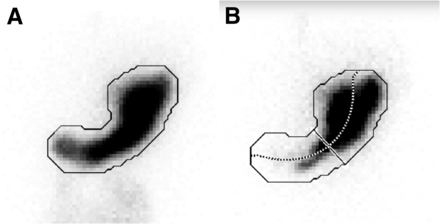
IMD was quantitatively measured with Matlab semiautomated software, as previously described (6). This software first defines an ROI for the total stomach using a summation of all coregistered anterior static gastric images (Fig. 1A). The software then divides the stomach into proximal and distal halves along the longitudinal axis of the stomach. The longitudinal axis is found by taking the midpoints between the ROI that defines the inner curvature and outer curvature of the stomach, starting from the most distal stomach boundary and moving toward its most proximal point. A third-degree polynomial is used to smooth the midpoints and to define the longitudinal axis. The midpoint (50% of the distance along the longitudinal axis) is used as the reference point to divide the distal half from the proximal half of the stomach (Fig. 1B). These regions are separated by a line that goes through the reference point and is perpendicular to the longitudinal axis. Measurement of IMD0 using the proximal half of the stomach is defined as the ratio of counts in the proximal half of the stomach to the total gastric counts, immediately after meal ingestion.
Anatomic division of stomach into proximal and distal halves by semiautomated software. (A) Demonstration of how software automatically contours outer border for total-stomach ROI (solid line) from summed set of all static anterior images. (B) Application of total-stomach ROI to each static image. In this case, image is anterior image acquired immediately after meal ingestion. By finding midpoint between opposing points of total-stomach ROI, longitudinal axis through stomach is generated (dotted line). Line perpendicular to longitudinal axis is then defined at point dividing longitudinal axis into equal halves to separate upper and lower segments of stomach.
IMD Based on Partitioning of Proximal Stomach from Contracting Antrum (Distal Stomach) by DACS Fourier Analysis
We used the method of fast Fourier transformation to automatically differentiate the noncontracting proximal stomach from the distal contracting antrum. Fast Fourier transformation of the time–activity curve of counts from a vertical ROI in the midportion of the antrum was performed as previously described (16). Fourier analysis reveals which pixels demonstrate regular, rhythmic contractions and a dominant frequency. The gastric antrum was then defined as that portion of the stomach containing these contracting pixels. The remaining area of the stomach was specified as the noncontracting proximal stomach (Fig. 2).
Fourier frequency and amplitude analysis used to segment antrum (distal stomach) from proximal stomach based on antral contractions. (A) Fast Fourier transformation of all pixels in DACS image set: amplitude and frequency response. (B) Red border on amplitude response indicating threshold of high-amplitude pixels. Same border is applied to frequency response. (C) Region of dominant frequency. Green border indicates contiguous region of pixels that have same frequency (gray color). (D) Starting location of antral contractions. Antrum-defined ROI (green border) using region of dominant frequency from C and red border from B is applied to one image of gastric emptying study. Purple dotted line is longitudinal axis as shown in Figure 1B. Yellow X is intersection of longitudinal axis, and convex boundary around green border is starting location of antral contractions.
Additional values derived from the fast Fourier transformation DACS analysis included time of onset of sustained antral contractions after meal ingestion (Fig. 3), the location of the site of origination of antral contractions referenced to the long axis (Fig. 2C), the dominant frequency of contractions, the antral ejection fraction; and the speed of propagation of the antral contractions. The antral ejection fraction is a measure of the amplitude of antral contractions, defined by the percentage change in measured dynamic time–activity curve of the mid antral ROI created by the propagating contracting waves (supplemental appendix).
Time to onset of antral contractions. (A) Vertical rectangular ROI is placed over midportion of gastric antrum from one frame of serial anterior dynamic images used to generate time–activity curves shown in B. (B) y-axis is counts generated in antral ROI shown in A over time. x-axis is frame number, with 400 frames at 3 s per image. Time to onset of regular antral contractions is seen at image 278 or at 13.9 min (arrow) after immediately-after-meal static images.
Statistical Analysis
The results were entered into a Microsoft Excel database. Reported results are expressed as mean ± SD. The Student t test was used to test differences between values. Spearman correlation was used to correlate different parameters using the descriptors very weak (0.00–0.19), weak (0.20–0.39), moderate (0.40–0.59), strong (0.60–0.79), and very strong (0.80–1.0) (17).
RESULTS
Study Subjects
Twenty-one subjects were studied. One subject was excluded from the final analysis because of excessive motion artifacts during the DACS imaging sets that could not be adequately corrected. The final data analysis therefore included 20 subjects (average age, 24.6 ± 6.6 y; 7 female and 13 male).
Total Gastric Emptying
Initial preferential retention of solids in the proximal stomach (fundic accommodation) was seen in all subjects. Over time, all subjects showed progressive transit of the radiolabeled solid meal from the proximal to the distal stomach (Fig. 4).
(A) Total and regional gastric retention over time using anatomic division of stomach into halves. (B) Total and regional gastric retention over time using Fourier frequency and amplitude to separate antrum from remaining proximal stomach.
Total mean gastric retention is summarized in Table 1. Mean total gastric retention was 39.8% ± 12.8% at 2 h and 5.8% ± 6.0% at 4 h. These clinically used 2- and 4-h values for measuring gastric emptying were compared between the men and women. For the 13 men, retention was 37.7% ± 13.4% at 2 h and 4.0% ± 4.2% at 4 h, whereas for the 7 women, retention was 43.5% ± 11.3% at 2 h (P = 0.142 vs. men) and 8.8% ± 7.7% at 4 h (P = 0.142). The mean half-time for gastric emptying in all patients was 1.62 ± 0.33 h: 1.54 ± 0.32 h for the men and 1.75 h ± 0.28 h for the women.
Results Summary (n = 20 Healthy Subjects)
IMD Based on Division of Stomach into Proximal and Distal Halves
The regional percentage of solid meal retention at 0, 30, 60, 120, 180, and 240 min is shown in Figure 4A. The measure of fundic accommodation, IMD0, based on division of the stomach into halves averaged 0.75 ± 0.15 (Table 1). Over time, the IMD progressively decreased, representing movement of the radiolabeled meal from the proximal stomach into the distal stomach and subsequent gastric emptying.
DACS
Using DACS, the time of first onset of regular and sustained antral contractions was obtained. On average, sustained and periodic antral contractions started at a mean of 11.24 ± 12.98 min after meal ingestion. Figure 3 shows the typical conversion from irregular, nonperiodic to sustained, regular contractions.
Antral contraction frequency peaked during the DACS image set taken 30 min after meal ingestion, at 3.30 ± 0.71/min. This 30-min interval after meal ingestion was also the time when distal antral filling was the greatest (36.65% ± 13.49% based on proximal vs. distal division into halves and 25.98% ± 15.10% based on Fourier separation) (Figs. 4A and 4B). The ejection fraction also peaked during the DACS image set taken 30 min after meal ingestion (30.31% ± 13.69% ejection fraction).
The average speed of contractions from 0 to 80 min after meal ingestion was 3.16 mm/s. The mean location of the onset of antral contractions was in the midbody of the stomach, with the average location of onset of contractions being 40.54% ± 10.78% from the distal to the proximal stomach along the longitudinal axis.
IMD Based on DACS and Other Derived Measures of Antral Contraction
IMD0 calculated by segmenting the proximal stomach from the antrum by defining the antrum by those pixels demonstrating phasic antral contractions was 0.85 ± 0.14, which was greater than the mean value for IMD0 based on gastric division into halves (0.75 ± 0.15) (P = 0.004). There was a moderate correlation between IMD0 calculated by the two methods (r = 0.465; P < 0.05).
There were no significant sex differences between the male and female values for DACS-derived IMD0 (P = 0.13)—either for antral contraction frequency at 0 min (P = 0.34), 30 min (P = 0.48), or 60 min (P = 0.22) or for antral ejection fraction at 0 min (P = 0.13), 30 min (P = 0.34), or 60 min (P = 0.44).
DISCUSSION
The purpose of this study was to develop and obtain reference values for a GES study that goes beyond assessment of just total gastric emptying. Antral contractility was assessed in this study by adding continuous dynamic imaging or DACS after each static imaging set. IMD and DACS were analyzed using software developed in Matlab, which is readily available.
As in any first investigation of a new technique, we recognize that our results are limited to a small number of healthy subjects and will require validation and expansion to a larger number of healthy subjects. Because the healthy volunteers were all young (average age, 25 y), we are not able to look at age-related differences. There were no significant sex differences found for gastric retention or for the DACS-derived measurements of IMD0, antral contraction frequency, or ejection fraction.
We compared two measurements of IMD0, which assesses fundic accommodation. IMD is a reflection of regional gastric motility, with IMD immediately after meal ingestion (IMD0) being used to assess fundic accommodation (6). In this study, IMD0 using the proximal half of the stomach averaged 0.75 ± 0.15. We previously established a lower limit of 0.568 for normal IMD0 when the stomach is halved using receiver operating curves to define sensitivity and specificity in a large group of patients. In that study, also on several healthy subjects, IMD0 averaged 0.672 ± 0.092 (6). As shown in Table 1, the mean reference range for IMD0 using phasic contractions to define the antrum ranged from 0.71 to 0.99.
In this study, we assessed a potentially more physiologic method to measure IMD0 assuming that the stomach can be divided functionally by its phasic contractions into proximal and distal segments using DACS. This assessment is more physiologic than simple anatomic division of the stomach (18). The mean normal value for IMD0 obtained on the basis of DACS-derived antral contractions (0.85 ± 0.14) is higher than that using anatomic division of the stomach into halves (0.75 ± 0.15; P = 0.004) because of a smaller physiologic area of DACS-defined antral contractions in the distal stomach. The result is a larger defined area of proximal stomach. There was a moderate correlation between IMD0 calculated by the two methods.
Anatomic division of the stomach into halves has appeal because of its simplicity and ease of automation. We have shown in patients that measuring IMD0 using the proximal half of the stomach correlated best with increased early satiety (6). Using Fourier analysis and DACS to measure fundic accommodation response adds complexity to GES and will require further study in a larger patient population to determine whether it correlates better with symptoms and other physiologic tests of fundic accommodation.
DACS provides additional measures of antral contractility. The use of fast Fourier transformation permits measurements of both the frequency and the amplitude of contractions. The frequency of antral contractions was highest on average (3.30 ± 0.73/min) at 30 min after meal ingestion. The ejection fraction was also highest at 30 min, 29.43% ± 13.42%, suggesting that the most prominent contractions occur early, between 30 and 50 min after ingestion. The speed of contractions was highest 60 min after ingestion, and the average speed of antral contractions was 3.54 ± 0.90 mm/s at that time. Electrogastrography can also provide information on the frequency of gastric contractions (19). Although both DACS and electrogastrography can provide information on the frequency of antral contractions, electrogastrography does not provide information on the amplitude or strength of antral contractions or directly measure the speed of contraction.
The imaging protocol used in this study allows more complete characterization of proximal stomach fundic accommodation and distal stomach antral motility. Obtaining quantitative measures of fundic accommodation and antral contractility from GES images provides a quantitative and noninvasive method to provide more information on gastric motility and potential pathophysiology in symptomatic patients. Studies that assess fundic accommodation and antral contractility have already proven beneficial in explaining sex-related differences in gastric motility (16,20).
We recognize that adding measurement of IMD and impaired fundic accommodation adds time and complexity to the acquisition and processing of GES data. To answer whether this added complexity is worthwhile, one needs to understand the clinical limitations of solid-meal GES as currently performed. It has been shown that 25% of patients with functional dyspepsia sent for GES studies have a normal conventional gastric emptying result that fails to direct the ordering physician to a treatable cause of the patient’s symptoms (3). These patients are left without a diagnosis and potential for treatment. It is further estimated that approximately 40% of patients with functional dyspepsia have impaired fundic accommodation (11). Studies have shown that therapy with pharmacologic agents that relax the fundus can improve impaired fundic accommodation symptoms in these patients (21–23). Therefore, accurate measurement of IMD to assess for abnormal fundic accommodation as a cause of a patient’s symptoms can direct therapy for a significant number of patients. Other drugs have been shown to specifically increase antral contractility (24–26).
Several methods have been proposed for measuring IMD and impaired fundic accommodation as a part of a solid-meal GES study. These include simple visual assessment or quantification of the GES static images by division of the stomach into halves or thirds (6). It is unclear at this time whether a GES assessment of fundic accommodation with measurement of IMD should be done only after a normal GES result. A final determination of whether our proposed more physiologic method of defining the antrum by DACS and whether measurement of fundic accommodation is used before or after a normal GES study will require further study as a part of future larger clinical trials.
CONCLUSION
We have investigated a more comprehensive GES study combining both conventional static GES with DACS in healthy volunteers. This protocol provided assessment of IMD and antral contractility along with total gastric emptying and allows for a more physiologic division of the proximal versus the distal stomach. These reference results permit comparison to symptomatic patients to delineate potential gastric motility abnormalities causing patient symptoms. Broader clinical use will require further multicenter development of reference values and investigation of how this more comprehensive testing may improve diagnosis and aid targeted treatment of patients with upper gastrointestinal symptoms of dyspepsia and gastroparesis.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Aug. 23, 2018.
REFERENCES
- Received for publication June 1, 2018.
- Accepted for publication August 17, 2018.